In the last episodes, we examined the characteristics of dinosaurs and presented some evolutionary transitions within non-avian dinosaurs. But probably the most important and best documented evolutionary transition is the one between theropods, the carnivorous dinosaurs, and modern birds.
Within the dinosaur family tree, birds belong to the Avialae (Weishampel et al. 2004, Currie 2023, Fastovsky & Weishampel 2021, Holtz 2007, Paul 2016, Prothero 2017, 2022, Schweitzer et al. 2021, Neukamm & Beyer 2023, Cau 2018, Gauthier & Gall 2001, Norell & Xu 2005, Witmer 2009, Padian & Chiappe 1998, Chiappe 2009 Fig. 1). Paleontologists traditionally include all species descended from the last common ancestor of Archaeopteryx and modern birds in the Avialae – mainly small, airborne feathered animals with fully developed wings (Senter 2007, Agnolin & Novas 2013). Jacques Gauthier, who named the Avialae in 1986, redefined them in 2001 as all dinosaurs that possessed feathered wings used for flapping flight and the birds that descended from them (Gauthier 1986, Gauthier & de Queiroz 2001).
pdf file
The sister taxon of the Avialae are the Troodontidae, both clades together form the Averaptora. Averaptora and the dromeosaurids, including genera such as Deinonychus, Velociraptor or Microraptor, form the Paraves. And the Paraves together with the Oviraptorosaurs form the Pennaraptora. Pennaraptora plus Therizionsauridae and Alvarezsauroidea finally form the Maniraptora (“hand predators”). The Maniraptora and the Ornithomimosaurs form the clade of the Maniraptoriformes and these together with the Compsognathidae and the Tyrannosauroidae, to which the T. rex also belongs, form the Tyrannoraptora. The Tryannoraptora, together with some basal genera such as Zuolong, are then the Coelurosaurs (hollow-tailed lizards). The coelurosaurs are then again a subgroup of the theropods (Fig. 1). Today, only a very few dinosaur specialists and paleornithologists dispute this finding, and the few who do so seem to have ideological rather than scientific reasons (cf. Prum 2003, Smith et al. 2015, Rauhut & Foth 2020).
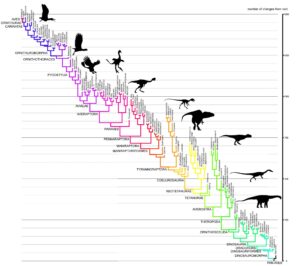
Fig. 1: Phylogenetic tree of Pan-Aves (Avemetatarsalia). This taxon (or clade) includes modern birds (Aves, top left), birds in a broader sense (basal Avialae), non-avian dinosaurs, pterosaurs, and other basal archosaurs more closely related to birds than to crocodilians. Modern birds differ from the stem species of Pan-Aves located at the base of this tree (lower right) by about 1500 morphological changes (derived traits). These acquisitions emerged successively within the ancestral lineage leading to modern birds over the last 250 million years. As expected, the groups are hierarchically nested. Thus, Dinosauria include Theropoda, Theropoda include Tetanurae, and Tetanurae include Coelurosauria. Maniraptora, in turn, is a subgroup of Coelurosauria. Pennaraptora is a subgroup of Maniraptora, and so on. Thereby, every node in this phylogenetic tree is a lineage-splitting event. The hierarchical system and the graded similarity of species contained in it are the strongest evidence for the evolution and descent of birds from early dinosaurs.. Illustration: James Paul Baello, modified after Cau (2018). For a higher resolution see: https://www.ag-evolutionsbiologie.net/bilder/kladogramm-pan-aves.jpg
Archaeopteryx
Among experts, the connection that birds are dinosaurs enjoys the status of a well-established theory. How did this come about? It actually started with an old feather.
In 1861, just 2 years after the publication of Darwin’s book, a remarkable specimen was found in the limestone quarries of Solnhofen in Bavaria. These quarries had been mined for years because they yielded beautiful flat slabs of extremely fine limestone that could be etched with acid to form the lithographic plates that printers used to make book illustrations. Occasionally, exquisitely preserved fossils have also been found in these limestones, including the tiny dinosaur Compsognathus and some of the first well-preserved pterosaurs (Fig. 2).
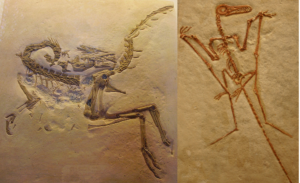
Fig. 2: left: Compsognathus, right: pterosaur from Solnhofen
But in 1860, an imprint of a fossil feather was found, and six months later workers came across the partial skeleton of a strange creature that had feathers but bones like a dinosaur (Fig. 3). This specimen naturally caused quite a stir, and the British Museum in London outbid all others to acquire it. Once it arrived in London (it is still known as the “London” specimen because it is there), it was the job of Richard Owen, the curator of the British Museum and the man who named the “Dinosauria”, to describe it. It had already been given the name Archaeopteryx (“old wing”), and although Owen basically described it as a bird, he couldn’t help but recognize all the dinosaur features of the skeleton. However, as he was one of the last serious biologists to oppose evolution, he made no effort to link this fossil to its relatives.
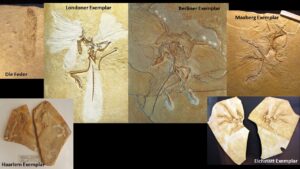

Fig. 3: All Archaeopteryx specimens
However, the dinosaur characteristics did not escape Owen’s rival Thomas Henry Huxley, who by this time had become “Darwin’s bulldog”, giving speeches and publishing works supporting Darwin’s theory. As one of the first to carry out anatomical studies on modern birds and to study a number of dinosaurs such as Compsognathus, Huxley could not help but notice that Archaeopteryx was a classic “missing link” between birds and dinosaurs (Huxley 1868, 1870). In a famous lecture to the Royal Society in 1863, he proposed that birds were descended from dinosaurs and listed 35 features that only dinosaurs and birds shared (17 of these features are still used by modern paleontologists Padian & Chiappe 1998). In 1877, an even better fossil was found, the classic “Berlin specimen”, which is the best preserved of the 12 known specimens. By this time, the Germans had already recognized the importance of Archaeopteryx. German industrialists bought it and ensured that it remained in Berlin, where it is now on display in the Museum für Naturkunde. It even survived the bombing during the Second World War. I’ve seen both the London and Berlin originals, and it’s like a pilgrimage to the Holy Grail to see such amazing and historic fossils. In fact, the skeleton of Archaeopteryx is so strikingly similar to that of the predatory dinosaur Compsognathus that two seemingly featherless specimens of the prehistoric bird were mistaken for the non-bird theropod for decades (the Eichstätt and Solnhofen specimens; Shipman 1999, p. 43 f.).
Huxley’s hypothesis was temporarily sidelined due to an influential book by the Dutch paleontologist Gerhard Heilmann. Heilmann argued that theropods appeared to lack clavicles, which in birds are fused to form the furcula, so that they could not possibly be ancestors of birds (Heilmann 1926). He ignored all the inferred similarities between dinosaurs and birds that Huxley had demonstrated, mainly because most paleontologists considered dinosaurs to be large and specialized and could not imagine that birds were descended from these giant land animals. Heilmann’s thinking was also dictated by scenarios of how birds evolved flight by gliding from branch to branch (the “tree down” hypothesis), and large terrestrial dinosaurs do not fit this scenario. We also now know that most theropods already had furcula, and have done so since the Triassic (Rauhut et al. 2020). As these bones are quite fragile, they are rarely preserved. Let’s take a closer look at Archaeopteryx as a transitional fossil (Yalden 1984, Chiappe & Witmer 2002, Ostrom 1974,1976, 1985, Christensen & Bonde 2004, Longrich 2006, Prothero 2017, Fig. 4):
Like most theropod dinosaurs (but not living birds), it had a long bony tail, a heavily perforated skull with teeth, theropod (non-bird-like) vertebrae, a strap-like scapula, a pelvis midway between that of typical lizard pelvic dinosaurs and later birds, ventral ribs, and unique specializations on the limbs.
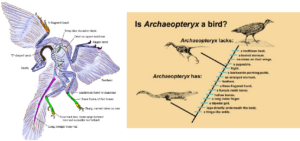
Fig. 4: Archaeopteryx
Most striking are the wrists (Fig. 5). Birds and some theropod dinosaurs, such as the dromaeosaurs, all have a semilunate wrist, which consists of the fusion of several wrist bones. It serves as the main hinge for wrist movement, allowing dromaeosaurs to extend their wrists and grasp prey with a quick back and forth pull. Coincidentally, the exact same movement is part of the downward flight stroke of birds. Archaeopteryx had the same three fingers (thumb, index and middle finger) as most other theropod dinosaurs, and the middle finger (the index finger) is by far the longest. In addition, the claws of Archaeopteryx are very similar to those of theropod dinosaurs.
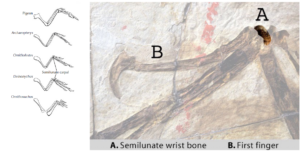
Fig. 5: Anatomy of the forelimbs of birds and dinosaurs, showing the semilunate wirst bone.
The hind limbs of Archaeopteryx also show many hallmarks of dinosaurs (Fig. 6). The most striking feature is the ankle. In the first part of this series we already learned about the mesotarsal joint as an apomorphy of dinosaurs and pterosaurs, which is of course also found in Archaeopteryx. In addition, part of the first row of tarsal bones has an a bony spur that runs up the front of the tibia (the ascending process of the astragalus), another unique feature of lizard-hipped dinosaurs and birds. Finally, the details and structure of the toe bones and big toe are also unique to theropods and birds, although Archaeopteryx did not have an opposable big toe that would have allowed it to grip branches well.
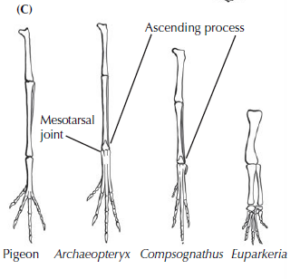
Fig. 6: Anatomy of the hind leg of birds and dinosaurs, showing the mesotarsal joint and the ascending process of the astragalus.
In fact, it has only a few bird-like features not found in other theropods: The big toe is fully inverted, the teeth are unserrated, and the tail is relatively short, but the arms are long compared to most other theropods. All other features of Archaeopteryx, including the feathers and the furcula, have since been found in other theropod dinosaurs (Figs. 4 & 7).
Fig. 7: Comparison between Archaeopteryx and its close relative Velociraptor, showing some of the key anatomical similarities that suggest birds evolved from other dinosaurs. In addition to these skeletal similarities, some of the non-bird dinosaurs have been shown to have had feathers.
In light of all this overwhelming evidence, it is bizarre to read how the creationists misrepresent Archaeopteryx. In their view, created “kinds” must be distinct, and transitional forms cannot exist, so they will do whatever it takes to discredit Archaeopteryx as a transitional form. Because Archaeopteryx had feathers, they believe it must be a plain bird. The typical dinosaur characteristics mentioned, which modern birds do not have, are ignored. An example of this amateurish approach is the American young-earth creationist Duane Gish, who was a luminary in the field of creationist absurdities during his lifetime (Gish 1995). He argues that the teeth of Archaeopteryx do not resemble those of theropods, but some extinct “toothed birds”. While this is not true either, they have both primitive similarities to theropod teeth and their own derived features, this whole argument misses the point: no living birds have teeth, but if fossil birds like Archaeopteryx did, then that links birds and dinosaurs. He also mentions the long tail of Archaeopteryx and counters that some reptiles and some birds have long tails and some have short tails. The point is that no living bird has a long bony tail. Gish tries to discredit the three bony clawed fingers of Archaeopteryx by pointing to the hoatzin, which also have these three fingers while they are still chicks (although he doesn’t mention that their configuration is completely different; Fig. 8). But an isolated atavism does not change the fact that the hand of Archaeopteryx is basically a dinosaur. No other living bird except hoatzin chicks has this kind of hand, which is highly specialized and in no way resembles Archaeopteryx’s hand. In short, every time creationists mention a feature that makes Archaeopteryx a dinosaur, they twist the evidence, show their ignorance of the anatomical details, or fail to mention counter-arguments or details that would discredit their case.

Fig. 8: Hoatzin (Opisthocomus hoazin)
The renaissance of dinosaur-bird evolution
But Archaeopteryx is by no means the only example of the connection between dinosaurs and birds! Huxley’s hypothesis experienced a renaissance through publications by the paleontologist John Ostrom, who showed that birds share more characteristics with theropods than with any other archosaur group (Ostrom 1976). He was influenced by the fact that in the 1960s he had discovered and described the highly specialized dinosaur Deinonychus from the family Dromeosauridae, which shows astonishing anatomical similarities with the earliest birds (Ostrom 1969, Fig. 9).
Ostrom’s conclusion that birds descended from small theropods became increasingly accepted as phylogenetic systematics became the standard in comparative biology. Gauthier (1986) described 84 commonly derived features of Saurischia dinosaurs, including theropods, and fully confirmed Ostrom’s conclusion. Using cladistics, he showed that the birds belong to the maniraptors, these to the coelurosaurs and these in turn to the theropods.
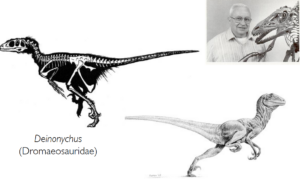
Fig. 9: In the 1960s, the palaeontologist John Ostrom put forward the idea that birds are descended from theropod dinosaurs. The dinosaur he described, Deinonychus, exhibits a number of characteristics that are also found in birds.
The data supporting this analysis has increased considerably, as has knowledge of the successive acquisition of bird traits (Brusatte et al. 2015, Smith et al. 2015, Cau 2018, Rauhut & Foth 2020). One example:
In the 1990s, expeditions from the American Museum of Natural History made some remarkable discoveries in the Gobi Desert in Mongolia, including nests containing eggs of the dinosaur Oviraptor. These eggs were so common in Mongolia that the original American Museum expeditions in the 1920s had attributed them to the most common dinosaur of these strata, the horned dinosaur Protoceratops (Osborn 1924, Coombs 1989, Thulborn 1992). When the bones of a small theropod were found near some of the nests, they were given the name Oviraptor (“egg thief”). But recent expeditions show that this name is slanderous: Oviraptor did not steal the eggs – it was their mother! In some cases, the female Oviraptor skeleton was buried in brooding posture directly over the eggs when both were buried and fossilized in a sandstorm. The details of this brooding posture and the way it was preserved show that many theropod dinosaurs behaved more like birds than reptiles (Norell et al. 1994, 1995, Dong & Currie 1996, Hopp & Orsen 2004, Clark et al. 1999, Sato et al. 2005, Varricchio et al. 2008, Yang et al. 2018, 2019; Fig. 10).

Fig. 10: Fossil finds of the oviraptosaur Citipati, evidence that dinosaurs incubated their eggs.
The most staggering discoveries come from the famous Lower Cretaceous Liaoning fossil deposits in China, which are now among the most important fossil deposits in the world. Extraordinary fossils are preserved in these fine lake shales, including body outlines, feathers and complete skeletons with not a single bone missing. Over the past 20 years, an important new find from these deposits has been announced every few months, and almost all previous ideas about birds and dinosaurs have been quickly overtaken by these discoveries (see Norell 2005 for a summary). The most amazing fossils of all were a number of clearly non-flying, non-bird dinosaurs with well-developed feathers. These include incredibly complete specimens such as Sinosauropteryx, Protarchaeopteryx, Sinornithosaurus, Caudipteryx, Beipiaosaurus and Microraptor. (Ji & Ji 1996, 1997, Xu et al. 1999b, Ji et al. 1998, Xu et al. 1999a, 2000; Figs. 11-16). Most of these fossils clearly do not have flight feathers or other indications that their feathers were used for flight.Instead, they show that feathers appear to have been widespread in theropods (and perhaps other dinosaurs and archosaurs, especially pterosaurs). So feathers did not evolve for flight, but were already present in theropods, presumably for insulation, and later became flying structures.
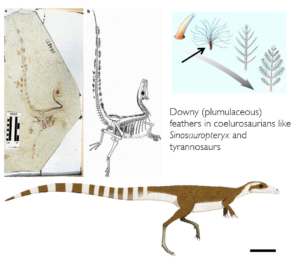
Fig. 11: Sinosauropteryx

Fig. 12: Protarchaeopteryx

Fig. 13: Sinornithosaurus
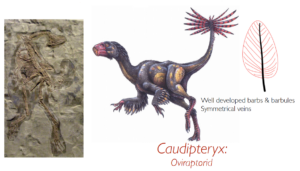
Fig. 14: Caudipteryx
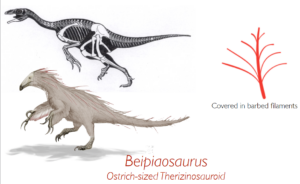
Fig. 15: Beipiaosaurus

Fig. 16: Microraptor
As already emphasized, the existence of (proto-)feathers in non-bird dinosaurs was also predicted on the basis of the theropod affiliation of birds. For example, the ornithologist Richard Prum (1999) predicted a series of evolutionary intermediate forms of feathers based on embryological differentiation processes in birds (after Sues 2001, Prum 1999, Prum & Brush 2003, Perrichot et al. 2008, Fig. 17):
Stage 1 proposes an unbranched, hollow filament that develops from a cylindrical invagination of the epidermis around a papilla. The feather emerges at the base of the follicle through the continuous division of keratin-forming cells. The growth zone forms a follicle collar, from which the cells push out. Stage 2 involves the differentiation of the follicle collar into barb ridges; a tuft of unbranched filaments emerges. Stage 3a represents the formation of a central rachis (shaft) via fusion of barbs and the development of a planar feather with unbranched barbs. Stage 3b displays the development of barbules that branch from the tufts of barbs; this corresponds morphologically to a downy feather. In stage 3a+b, the features of stages 3a and 3b combine to produce a planar feather with a central rachis, secondary branched barbs (barbules stem from the barbs), and an open vane. In stage 4, the barbules differentiate into hooklets and bow barbules, generating a closed pennaceous vane. Finally, in stage 5, lateral displacement of the new barb locus by differential new barb ridge addition to each side of the follicle leads to the growth of a closed pennaceous feather with an asymmetrical vane resembling modern remiges.
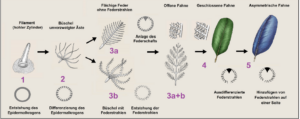
Fig. 17: development of feathers after Prum (1999) and Prum & Brush (2003).
If the theropod hypothesis is correct, then certain feather stages that are passed through in the ontogeny of birds should have existed as fully developed feather subtypes in adult dinosaurs. The rest, as they say, is history: Gradually, all (!) of the feather subtypes predicted from Prum’s ontogenetic model were discovered on theropod skeletons or in amber.
Type 1 feathers are found in Sinosauropteryx. Type 2 and type 3 feathers are found in the large therizinosaur Beipiaosaurus, suggesting that they were present in almost all theropods. Type 4 feathers are found in Caudipteryx, suggesting that they were also present in higher theropods (including Tyrannosaurus rex). The classic asymmetrical flight feather with the shaft near the leading edge of the wing first appears in Archaeopteryx, and for this reason many scientists believe that Archaeopteryx was one of the first to modify the long legacy of feathers for true flight (Prum & Brush 2003, Prothero 2017, Roy et al. 2020, Carrol et al. 2019, McKellar et al. 2011, Benton et al. 2019, Neukamm & Beyer 2023).
Another, not explicitly predicted intermediate form even mediates between stages 2 and 3a. Here, several branches are loosely connected at the shaft, which consists of incompletely fused secondary branches. The flattened, bilaterally symmetrical shape of modern feathers is already apparent (Neukamm & Beyer 2023).
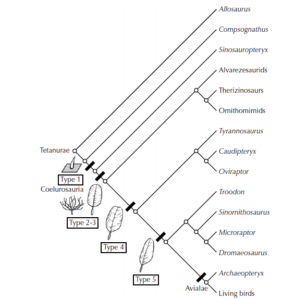
Fig. 18: The evolution of feather types from simple feather shafts to down feathers to complex flight feathers with asymmetrical plumes and shafts. Based on their occurrence in various feathered, non-flying dinosaurs from Liaoning, we can demonstrate that most predatory dinosaurs (including T. rex) probably had feathers of some kind.
Fig. 19: Fossil feathers in amber. The morphology of the specimen on the left corresponds to stage 3b of Prum’s widely accepted model (from: Roy et al. 2020). The center photo shows a feather in stage 3a (from: Caroll et al. 2019). Right: Stage 3a+b feather (from: McKellar et al. 2011; www.tinyurl.com/8h5edctm). Below: Another intermediate form of the feather preserved in amber. Here, several branches are loosely connected to the shaft, which consists of incompletely fused secondary branches. The flattened, bilaterally symmetrical shape of modern feathers is already apparent. The stage is intermittent between step 2 and step 3a in Prum’s model.
Another special representative is Rahonavis from the Cretaceous of Madagascar (Forster et al. 1998a, b, for taxonomic classification as Avialae see: Agnolin & Novas 2013, Cau 2018, Padian 2004, Chiappe & Dyke 2006, other authors consider Rahonavis outside the Avialae as Dromeosauridae, see Makovicky et al. 2005, Norell et al. 2005, Turner et al. 2007, Hartmann et al. 2019, Fig. 20). It is about the size of a crow, had the primitive sickle-shaped claws on the hind feet, the long, bony tail, teeth and many other theropod features, but also bird-like features such as the fusion of the lower dorsal vertebrae with the pelvis (the synsacrum), holes in the vertebrae for all the blood vessels and air sacs found in living birds, fingers with quill knobs, indicating that it was feathered, and a fibula that did not extend to the ankle. In birds, the fibula is reduced to a tiny sliver of bone, whereas Archaeopteryx has a fully developed fibula like the dinosaurs.
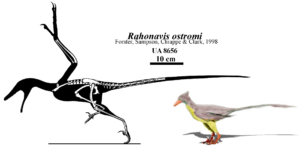
Fig. 20: Rahonavis
Avialae
The next step (Fig. 21) is marked by Confuciusornis and its relatives, which exhibit a unique feature found in all higher birds: the pygostyle, which was formed by the fusion of all ancient dinosaur tail vertebrae (Hou et al. 1995, Chiappe et al. 1999, Zhou & Hou 1998, Zhou 2004; Fig. 22). In Confuciusornis, seven dorsal vertebrae (sacral vertebrae) are fused to form the synsacrum, i.e. the section of the spine that forms the roof of the pelvis as a rigid structure. The bones that reinforce the shoulder are also elongated, which improves flight. They are also the first birds with a toothless beak.
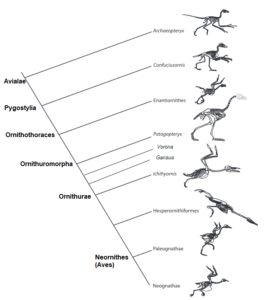
Fig. 21: Cladogram of Avialae
Fig. 22: Fossil and reconstruction of Confuciusornis; at the bottom right is a depiction of the synsacrum and pygostyle of modern birds.
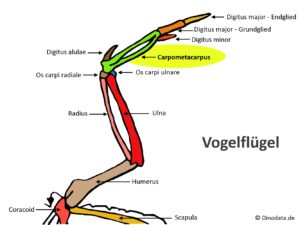
After this transitional form, there is a further branch that leads to the extinct Enantiornithes, a group of tooth-bearing birds that became extinct at the end of the Cretaceous (Walker 1981, Chiappe 2002, Chiappe & Walker 2002, Padian 2004, Zhou 2004, Zheng 2012, Wang et al. 2015, 2022, Fig. 23). In the Upper Cretaceous, they were the most species-rich group of Avialae and thus also more common than the “modern birds”, the Neornithes. These include Iberomesornis from Las Hoyas in Spain (Sanz & Bonaparte 1992), Sinornis from China (Sereno & Rao 1992), Gobipteryx from Mongolia (Elżanowski 1974), Enantiornis from Argentina (Walker 1981) and several others; in total, over 100 genera have been described. All these birds are more specialized than Archaeopteryx, Rahonavis or Confuciusornis in that they have reduced the number of trunk vertebrae, have a flexible furcula, have optimized the shoulder joint for flying, have fused the hand bones into a bone called carpometacarpus and the finger bones into a single element.
Fig. 23: Enantiornithes
They differ from modern birds in the articulation between the scapula and coracoid bone: In contrast to the arrangement in modern birds, the scapula has an articular socket and the coracoid bone has an articular peg (Fig. 24). In the carpometacarpus (Fig. 25) of Enantiornithes, the third metacarpal bone extended outwards beyond the length of the second metacarpal bone.
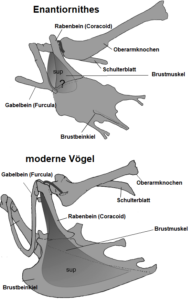
Fig. 24: Differences of the shoulder girdle in Enantiornithes and modern birds.
Fig. 25: Carpometacarpus of modern birds.
Due to their toe proportions and long curved claws, many Enantiornithes are considered to be arboreal. Some forms, such as Eoenantiornis (Hou et al. 1999, Zhou et al. 2005), Protopteryx (Zhang & Zhou 2000) and Eoalulavis (Sanz et al. 1996), had an alula (Fig. 46) and may have been better fliers than primitive birds such as Archaeopteryx, Jeholornis and Confuciusornis. A special find became known in June 2017 (Fig. 26): A foot and parts of the wing of a 99-million-year-old juvenile had been discovered in an almost eight-centimeter piece of amber in Burma (Xing et al. 2017, cf. also Xing et al. 2016, 2018, 2019a, b, 2020). There is also a 127-million-year-old chick from Spain, which was described in 2018 (Knoll et al. 2018).

Fig. 26: Chick found in amber.
It is particularly striking that young were found in many Enantiornithes genera. More detailed investigations revealed that the chicks of Enantiornithes were mostly precocial and that their eggs showed less variability in size than those of modern birds. However, the find from Spain proves that this was altricial (Fig. 27). This combination of tree dweller and precocial is unique, as the chicks of all modern tree-dwelling birds are altricial. The relatively early timing of reproductive maturity and their lower growth rate are also atypical for modern birds. Due to their delayed growth, the Enantiornithes were thus also exposed to the danger of predators for longer, which increased their mortality (Schmitt 2023).

Fig. 27: The chick of an enantiornith discovered in Spain (Knoll et al. 2018).
Enantiornithes were diverse in their diet: some had robust jaws for eating hard-shelled invertebrates. Others had short, blunt teeth and were probably used to feed on soft-bodied arthropods (O’Connor & Chiappe 2011) Other genera had strongly curved claws, suggesting that they preyed on small to medium-sized vertebrates, but their robust teeth are more indicative of a diet of hard-shelled animals (Wang et al. 2014).
A study of the digestive system shows that the known enantiornithes did not have a crop or gizzard, did not use gastroliths, and did not eject pellets. This is at odds with the wide diversity of diets resulting from their different teeth and skull shapes (O’Connor et al. 2019), although some modern birds have lost the gizzard and rely solely on strong stomach acids (Houston & Copsey 1994). One specimen was discovered that was thought to have gastroliths in the stomach of the fossil, which reopened the debate on the use of gastroliths by Enantiornithes. X-ray and scanning microscopic examination of the rock revealed that it was in fact chalcedony crystals and not gastroliths (Fig. 28)[1].
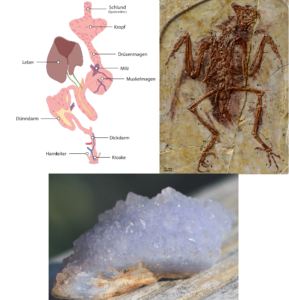
Fig. 28: Top left: Drawing of the digestive system of modern birds. Enantiornithes appear to lack a gizzard and crop. They also do not use stomach stones (gastroliths). Top right: The gastroliths discovered in the stomach of a specimen of Enantiornithes were chalcedony crystals (bottom).
The Longipterygidae (Zhang et al. 2001, Fig. 29) are the most intensively studied family in terms of diet due to their rather unusual rostral anatomy with long jaws and few teeth arranged at the ends of the jaws. They have been variously interpreted as piscivores (O’Connor et al. 2011), shorebirds (Hou et al. 2004) and tree-bark eaters (Morschhauser et al. 2009). However, a study from 2022 concludes that they are most likely generalist insectivores (Miller et al. 2022, Clark et al. 2023). Despite their diversity of dietary types, the Enantiornithes have not reached the range of modern birds, so there are no forms here that are adapted to life in water (Schmitt 2023).

Fig. 29: Longipterygidae
Further up the cladogram (Fig. 21), within the Euornithes group, we encounter several birds from the Cretaceous such as Vorona from Madagascar (Fig. 30; Forster et al. 1996; some authors classify it as Enantiornithes; see Pei et al. 2020), Patagopteryx from Argentina (Fig. 31; Alvarenga & Bonaparte 1992), Gansus (Fig. 32; Hou & Liu 1984) from China and the well-known waterbirds Hesperornis (Fig. 33; Marsh 1872a) and Ichthyornis (Fig. 34; Marsh 1872b) from the Cretaceous rocks of Kansas. These birds are linked by at least 15 clearly defined features, including the loss of the ventral ribs or gastralia, the realignment of the pubis into the modern bird-like position parallel to the ischium, the reduction in the number of trunk vertebrae, and many other features on the hand and shoulder that improved flight performance. Ichthyornis is even more similar to modern birds in that it had a keel on the sternum for the flight muscles and a button-like head on the humerus that made the wing more flexible. The group, which includes all modern members of the class Aves, is characterized by the complete loss of teeth and a number of other anatomical specializations, such as the fusion of the leg bones into a tarsometatarsus.
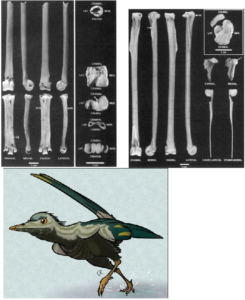
Fig. 30: Vorona

Fig. 31: Patagopteryx

Fig. 32: Gansus

Fig. 33: Hesperornis

Fig. 34: Ichthyornis
When modern birds diversified is not yet fully understood. Most studies agree that the most recent common ancestor of modern birds dates from the Cretaceous, but estimates range from the Early Cretaceous (Yonazawa et al. 2017, Lee et al. 2014) to the latest Cretaceous (Prum et al. 2015, Kuhl et al. 2020). Similarly, there is no consensus on whether most of the early diversification of modern birds occurred in the Cretaceous and was related to the breakup of the Gondwana supercontinent or later and possibly as a result of the Cretaceous-Paleogene extinction event (Ericson et al. 2006). Most molecular dating studies point to an evolutionary radiation in the Cretaceous, while fossil evidence points to a Cenozoic radiation. However, due to the rarity of fossilization, molecular data is likely to have a certain significance.
The discovery of Vegavis from the Maastrichtian, the last stage of the Late Cretaceous, proved that the diversification of modern birds began before the Cenozoic (Fig. 35; Clarke et al. 2005, Agnolin et al. 2017). Vegavis is classified as related to the family of geese and ducks, a family of modern birds that split off relatively early. The relationship of an earlier fossil, Austinornis lentus, dated to about 85 million years ago (Fig. 36; Clarke 2004), is still too controversial to provide fossil evidence for the diversification of modern birds. Some place it in the family of chicken birds, also an early group of modern birds. In 2020, Asteriornis was described from the Maastrichtian, it also appears to be a close relative of chicken and duck birds (Fig. 37; Field et al. 2020).

Fig. 35: Vegavis

Fig. 36: Austinornis lentus
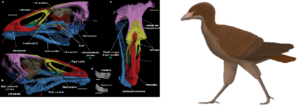
Fig. 37: Asteriornis
A 2015 estimate using a new method for calibrating molecular clocks confirmed that although modern birds evolved early in the Late Cretaceous, probably in western Gondwana, there was a diversification pulse in all major groups around the Cretaceous-Paleogene extinction event (Claramunt & Cracraft 2015). The most recent work shows that the most important groups (Paleognathae, Galloanserae and Neoaves) diverged between 100 and 75 million years ago (e.g. Field et al. 2020; Fig. 38).
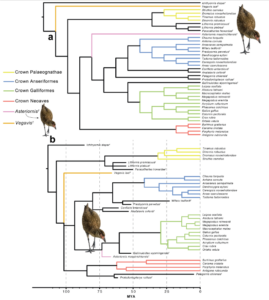
Fig. 38: a, Results of the Parsimony analysis. Asteriornis (pink) is resolved as a sister taxon of the Crown Galloanserae. b, Results of the Bayesian analysis with an age of 86.5 million years for Neornithes (after Field et al. 2020).
Mei long, Anchiornis, Mononykus, Fujianvenator: Bird oder Dinosaur?
Mei long, which translates as the sleeping dragon, is a non-avian dinosaur from the Troodontidae family, the dinosaur family that is the closest relative of the Avialae. It lived around 125 million years ago and was discovered in China. The type fossil is a complete and exceptionally well-preserved juvenile, about 53 centimeters long, with three-dimensional details, with the snout under one of the front legs and the legs folded neatly under the body, similar to the sleeping position of modern birds. This posture represents another behavioral link between birds and dinosaurs (Fig. 39; Lü et al. 2010, Xu & Norell 2004).

Fig. 39: The sleeping position of Mei long is similar to that of modern birds.
Also worth mentioning are the Anchiornitidae, whose best-known genus is Anchiornis. Anchiornis translates roughly as “almost-bird”. Fossils of Anchiornis have only been found in rocks from the late Jurassic period in China around 160 million years ago. It is known from hundreds of specimens, and given the excellent preservation of some of these fossils, it became the first Mesozoic dinosaur species for which almost the entire life history could be determined, and an important source of information on the early evolution of birds. Remains of the color pigments of some specimens are even known. It had a grey base color, a mohawk-like, reddish-brown crest on the head and white contour feathers with black bands on the front and hind legs. The discovery of Anchiornis is particularly significant because it represents a bird-like dinosaur that is older than Archaeopteryx (Fig. 40; Xu et al. 2009, Hu et al. 2009, Foth & Rauhut 2017, Li et al. 2010, Lindgren et al. 2015).

Fig. 40: Anchiornis
Its exact taxonomic position is debated in the scientific community. The anchiornithids have been classified in different places in the family tree of maniraptors, with some scientists classifying them as a separate family, as a basal subfamily of the Troodontidae (Xu et al. 2011, Lee & Worthy 2011, Brusatte et al. 2014, Shen et al. 2017) as members of the Archaeopterygidae (Hartmann et al. 2019) or as a group of dinosaurs that represent an evolutionary stage within the Avialae (Wang et al. 2016) or the Paraves (Lefevre et al. 2017).
Its ambiguous classification is due to the mosaic of features of Anchiornis and related genera of its family. Its skull shows features of dromeosaurs, troodontids and avialae. The postcranial skeleton, i.e. the skeleton behind the skull, is similar to that of troodontids. However, the forelegs were longer, similar in length to those of dromaeosaurids and basal birds. The forearm had ten long wing feathers, the hand had eleven, the lower leg 12 to 13, the foot ten to eleven. Hand and arm wings were about the same length. In contrast to the proportions in Archaeopteryx and Microraptor, the broader part of the wing of Anchiornis was proximal (towards the center of the body, Fig. 41). In addition to the wing feathers on the limbs, Anchiornis had two other types of feathers. Down-like tufted feathers whose filaments all converged at the base and contour feathers whose filaments were arranged along a long and solid quill (Xu et al. 2009, Hu et al. 2009, Longrich et al. 2012).
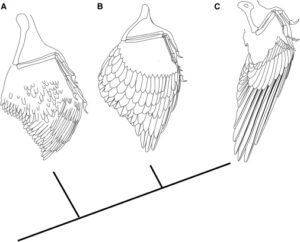
Fig. 41: Hypothetical evolution of the bird wing. (A) The original wing morphology (using Anchiornis as an example), in which the wing consists of slender, symmetrical and poorly differentiated flight feathers. (B) Wings of the early Avialae (e.g. Archaeopteryx), in which the flight feathers are elongated, broad and asymmetrical. (C) Wings of the Pygostylia (here Confuciusornis), in which the primary remigia are elongated and the covering feathers are shortened. Phylogeny according to Turner et al. (2012)
Another spectacular fossil is Mononykus, which lived around 70 million years ago. The fossil was found in 1987 in the Nemegt Formation in Mongolia and was described as a species in 1993. The name refers to the animal’s very short, stocky arms, in which the hand bones each end in a single phalanx that bore a thick, sharp claw. For a while, researchers could not even decide whether Mononykus was still a carnivorous non-avian dinosaur or a very early flightless ratite. In fact, it was first described as a ratite because, among other things, it has the keeled breastbone described for birds that previously flew alone. Such a bony ridge on the breastbone serves as an attachment surface for the powerful flight muscles in birds. In flightless birds such as ostriches and emus, the sternum keel is strongly reduced – similar to Mononykus. Other bird-like features were the soft palate, the foramen magnum, the neck and tail vertebrae and many others. Mononykus, together with similar genera, was placed in the family Alvarezsauridae as part of the Avialae (Fig. 42; Perle et al. 1993a, b, 1994).
Meanwhile, Mononykus, or the Alvarezsauridae, is no longer considered a secondary flightless ratite or Avialae, but is classified as a non-avian dinosaur within the Maniraptoriformes. These erroneous classifications of Alvarezsauridae as birds have been caused primarily by features that are strikingly or even uniquely avian. However, the earlier forms of the monophyletic group Alvarezsauridae do not have these avian features. The closest relatives of this dinosaur family are the Ornithomimosauria, which include famous genera such as Gallimimus from Jurassic Park. The remaining similarities between birds and alvarezsaurs, such as the keeled sternum, are another case of homoplasy, where the bird-like features have arisen through convergent evolution (Sereno 1991, Agnolin et al. 2012, Xu et al. 2011, Zhou 1995).
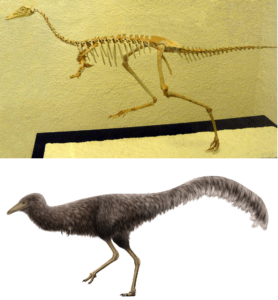
Fig 42: Mononykus
Incidentally, feathers have also been found in many ornithomimosaurs (Fig. 43). Clear evidence of feathers is known from Ornithomimus edmontonicus, of which there are several specimens showing traces of feathers (van der Reest et al. 2016). Feathers have also been speculated in Deinocheirus and Pelecanimimus; in Deinocheirus due to the presence of a pygostyle (Lee et al 2014) and in Pelecanimimus due to possible imprints (Briggs et al 1997).

Fig. 43: Some representatives of the Ornithomimidae with plumage.
Another family whose exact position in the phylogenetic tree of Maniraptora is not entirely certain are the Scansoriopterygidae from China (Fig. 44 Czerkas & Yuan 2002). Some authors classify them within the Avialae (Senter 2007, Zhang et al. 2008), others outside as basal Paraves or basal Pennaraptora (Agnolin & Novas 2011, 2013, Brusatte et al. 2014, Lefevre et al. 2014, Sorkin 2021, Pittman et al. 2020). They will be mentioned again in a separate article.

Fig. 44: Scansoriopteryx
The difficulty researchers have in distinguishing between Cretaceous birds and non-avian dinosaurs once again highlights how closely related these groups obviously are.
In 2023, a new feathered dinosaur genus was discovered: Fujianvenator prodigiosus, a pheasant-sized dinosaur with long legs and clawed wings from the Late Jurassic (Fig. 45; Xu et al. 2023). The new species, which is thought to have lived around 150 million years ago, has a bizarre combination of features that it shares with other representatives of the Avialae, Troodontidae and Dromaeosauridae. Particularly striking is the fact that the tibia is twice as long as the femur. Fujianvenator is thus characterized by a unique combination of the skeletal features of various archosaurs, which is why the first describers concluded that mosaic features in early theropod dinosaurs must have played an important role in the evolution of birds. The surprisingly long lower leg and other appearance features combined with geological observations indicated that Fujianvenator lived in a swamp-like environment and was a high-speed runner or long-legged wading bird. This finding contrasts with other early bird species, which are thought to have been more arboreal and airborne.

Fig. 45: Fujianvenator
Summary and Conclusion
Let us summarize:
Fig. 1 shows the phylogenetic tree of the Avemetatarsalia. In addition to modern birds (AVES, top left), birds in the broader sense (Avialae), non-avian dinosaurs and pterosaurs, this taxon includes all archosaurs that are more closely related to birds than to crocodilians. The higher birds are characterized by about 1500 morphological changes (Cau 2018) compared to the ancestral species at the base of the tree (bottom right). These occurred successively in the ancestral lineage of the birds. As expected, the groups are hierarchically nested within each other. The hierarchical system and the graded similarity of the species reflected in it is the strongest evidence for the evolution and descent of birds from early dinosaurs.
Another study found that a clear demarcation between birds and their immediate “non-avian” ancestors is not recognizable at all (Brusatte et al. 2014). For this study, data sets for more than 850 skeletal features were collected from around 150 theropods from that time period. Using a statistical method that takes many features into account, each species was mapped in a so-called morphospace (Fig. 46). In principle, this is a multidimensional map on which the species are grouped along several axes according to the proportion of characteristics they share. Two very similar species are then close together, while species that are different in many ways are far apart. If birds had evolved from dinosaurs as a result of a number of rapidly successive drastic mutations, i.e. if a completely different animal form had developed within a short period of time, then the two groups of theropods would have to occupy clearly different areas on the map and have their own morphospaces. However, this is not the case. Rather, the birds of that time are scattered colorfully in the middle of the cloud of dinosaurs. This means that they evolved so slowly that the transition became blurred.
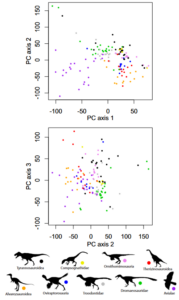
Fig. 46: Discrete morphospace of coelurosaurs. Representation of the overall anatomical variability of the species. Bivariate representations of the main coordinate axes 2 versus 1 and 3 versus 2 are shown (axes 1-3 account for 4.25% of the total variance). Birds differ largely from other coelurosaurs on principal coordinate axis 1, but not on axis 2 or 3 (or all subsequent axes). Permutation tests show that there is no clear, significant separation between birds and their closest theropod relatives.
In order to make sense of this morphological continuum, those theropod dinosaurs that lead to birds are defined as Avialae. However, the dromaeosaurids, including genera such as Deinonychus, Velociraptor and Microraptor, are not included, as their evolutionary lineage branched off shortly before.
More than 40 % of the key features of modern birds evolved during the 60 million year transition from the first bird-like archosaurs to the first maniraptors, i.e. the first dinosaurs that were closer to living birds than to Tyrannosaurus rex. After the appearance of maniraptoromorphs, the next 40 million years saw a continuous reduction in body size and the accumulation of neotenic (juvenile) features. Hypercarnivory became increasingly rare, while the cerebral hemispheres enlarged and the forelimbs became longer (Cau 2018). The integument evolved into complex, feathered plumes (Prum & Brush 2003, Prothero 2017, Roy et al. 2020, Carrol et al. 2019, McKellar et al. 2011, Benton et al. 2019, Neukamm & Beyer 2023).
During the Cretaceous, the Avialae evolved into a wide variety of forms. Many groups retained primitive features, such as clawed wings and teeth, although the latter were independently lost in a number of Avialae groups, including modern birds (Aves) (Chiappe 2007). Increasingly stiff tails are seen in the evolution of maniraptors, and this process culminated in the appearance of the pygostylus, an ossification of fused caudal vertebrae, as we can already see in Confuciusornis (Hou et al. 1995, Chiappe et al. 1999, Zhou & Hou 1998, Zhou 2004, Cau 2018). In the Late Cretaceous, around 100 million years ago, the ancestors of all modern birds evolved a more open pelvis, which allowed them to lay larger eggs relative to their body size (Pickrell 2018).
Finally, with the evolution of ornithothoraces, which include the Enantiornithes, Ichthyornis, Hesperornis and modern birds, there was a refinement of aerodynamics and the ability to fly, as well as the loss or fusion of several skeletal features. Of particular importance are the development of an enlarged, keeled sternum and alula (Fig. 47) and the loss of prehensile hands (Cau 2018).
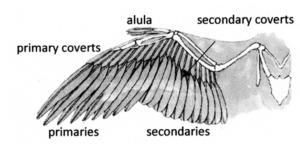
Fig. 47: Alula
There is therefore no way around the realization that birds have dinosaur ancestors – at least if one accepts scientific standards of rationality (cf. Prum 2003, Havstad & Smith 2019). This has been the scientific consensus for 20 years. In the next episode, however, we will address some of the objections raised by opponents of the dinosaur-bird relationship. It makes sense to deal with them insofar as they spread popular myths about evolution. We will also learn more about the evolutionary history of dinosaurs.
Literatur
Agnolín, F. L., Egli, F. B., Chatterjee, S., Marsà, J. A. G (2017): Vegaviidae, a new clade of southern diving birds that survived the K/T boundary. The Science of Nature. 104 (87): 87
Agnolín, F. L., Novas, F. E. (2011). Unenlagiid theropods: are they members of the Dromaeosauridae (Theropoda, Maniraptora)? Anais da Academia Brasileira de Ciências. 83 (1): 117–162.
Agnolín, F. L., Novas, F. E. (2013): Avian ancestors. A review of the phylogenetic relationships of the theropods Unenlagiidae, Microraptoria, Anchiornis and Scansoriopterygidae. SpringerBriefs in Earth System Sciences: 1–96.
Agnolin, F. L., Powell, J.E., Novas, F. E., Kundrát, M. (2012): New alvarezsaurid (Dinosauria, Theropoda) from uppermost Cretaceous of north-western Patagonia with associated eggs. Cretaceous Research. 35: 33–56.
Alvarenga, H. M. F., Bonaparte, J. F. (1992): A new flightless landbird from the Cretaceous of Patagonia. Los Angeles County Museum of Natural History, Science Series 36:51-64
Benton, M. J., Dhouailly, D., Jiang, B., McNamara, M. (2019): The Early Origin of Feathers. Trends in Ecology & Evolution. 34 (9): 856–869.
Briggs, D. E. G., Wilby, P. R., Pérez Pérez-Moreno, B., Sanz, J. L., Fregenal-Martinez, M. (1997): The mineralization of dinosaur soft tissue in the Lower Cretaceous of Las Hoyas, Spain. Journal of the Geological Society of London, 154: 587-588.
Brusatte, S. L., Lloyd, G. T., Wang, S. C., Norell, M. A. (2014): Gradual assembly of avian body plan culminated in rapid rates of evolution across the dinosaur-bird transition. Current Biology. 24 (20): 2386–2392.
Brusatte, S. L., O’Connor, J. K., Jarvis, E. D. (2015): The origin and diversification of birds. Current Biology, 25, R888–R898.
Caroll, N. R., Chiappe, L. M., Bottjer, D. J. (2019): Mid-Cretaceous amber inclusions reveal morphogenesis of extinct rachis-dominated. Scientific reports, 9, 18108.
Cau, A. (2018): The assembly of the avian body plan: A 160-million-year long process. Bollettino della Società Paleontologica Italiana, 57, 1–25.
Claramunt, S., Cracraft, J. (2015): A new time tree reveals Earth history’s imprint on the evolution of modern birds. Sci Adv. 1 (11): e1501005.
Clark, A. D, Hu, H., Benson, R. B., O’Connor, J. K. (2023): Reconstructing the dietary habits and trophic positions of the Longipterygidae (Aves: Enantiornithes) using neontological and comparative morphological methods. PeerJ 11:e15139
Clark, J. M., Norell, M. A., Chiappe, L. M. (1999): An oviraptorid skeleton from the Late Cretaceous of Ukhaa Tolgod, Mongolia, preserved in an avianlike brooding position over an oviraptorid nest. American Museum Novitates (3265): 1−36.
Clarke, J. A. (2004): Morphology, phylogenetic taxonomy, and systematics of Ichthyornis and Apatornis (Avialae: Ornithurae). Bulletin of the American Museum of Natural History. 286: 1–179.
Clarke, J. A., Tambussi, C. P., Noriega, J. I., Erickson, G. M., Ketcham, R. A. (2005): Definitive fossil evidence for the extant avian radiation in the Cretaceous. Nature. 433 (7023): 305–308.
Coombs, W. P. (1989): Modern analogs for dinosaur nesting and parental behavior. In Farlow, J. O. (ed.). Paleobiology of the dinosaurs. Geological Society of America Special Papers. Vol. Geological Society of America Special Paper 238. Colorado: Boulder. pp. 21−54.
Chiappe, L.M. (2002): Basal bird phylogeny: problems and solutions. In: L.M. Chiappe and L.M. Witmer (Hrsg.): Mesozoic Birds: Above the Heads of Dinosaurs. 448–472. University of California Press, Berkeley.
Chiappe, L. M. (2007): Glorified Dinosaurs: The Origin and Early Evolution of Birds. Sydney: University of New South Wales Press.
Chiappe, L. M. (2009): Downsized Dinosaurs: The Evolutionary Transition to Modern Birds. Evo Edu Outreach 2, 248–256.
Chiappe, L. M., Dyke, G. J. (2006): The early evolutionary history of birds. Journal of the Paleontological Society of Korea. Bd. 22, Nr. 1, S. 133–151.
Chiappe, L. M., Shu-An, J., Qiang, J., Norell, M. A. (1999): Anatomy and systematics of the Confuciusornithidae (Theropoda:Aves) from the Late Mesozoic of northeastern China. Bulletin of the American museum of Natural History no.242 89pp.
Chiappe, L.M., Walker, C.A. (2002): Skeletal morphology and systematics of the Cretaceous Euenantiornithes (Ornithothoraces: Enantiornithes). In: L.M. Chiappe and L.M.Witmer (Hrsg.): Mesozoic Birds: Above the Heads of Dinosaurs. 240–267. University of California Press, Berkeley.
Chiappe, L. M. Witmer, L. M. eds. (2002): Mesozoic birds: above the heads of dinosaurs. Berkeley and Los Angeles, California: University of California Press.
Christensen, P., Bonde, N. (2004): Body plumage in Archaeopteryx: a review, and new evidence from the Berlin specimen. Comptes Rendus Palevol. 3 (2): 99–118.
Currie, P. J. (2023): Celebrating dinosaurs: their behaviour, evolution, growth, and physiology. Canadian Journal of Earth Sciences. 60(3): 263-293.
Czerkas, S.A., Yuan, C. (2002): An arboreal maniraptoran from northeast China. Pp. 63-95 in Czerkas, S.J. (Ed.), Feathered Dinosaurs and the Origin of Flight. The Dinosaur Museum Journal 1. The Dinosaur Museum, Blanding, U.S.A
Dong, Z., Currie, P. J. (1996): On the discovery of an oviraptorid skeleton on a nest of eggs at Bayan Mandahu, Inner Mongolia, People’s Republic of China. Canadian Journal of Earth Sciences. 33 (4): 631−636.
Elżanowski, A. (1974): Preliminary note on the Palaeognthous bird from the Upper Cretaceous of Mongolia Palaeontologia Polonica 30.
Ericson, P. G.P., et al. (2006): Diversification of Neoaves: integration of molecular sequence data and fossils. Biology Letters. 2 (4): 543–547.
Fastovsky, D. E., Weishampel, D. B. (2021): Dinosaurs: A Concise Natural History (4th ed.). Cambridge university Press, Cambridge.
Field, D. J., Benito, J., Chen, A., Jagt, J. W. M., Ksepka, D. T. (2020): Late Cretaceous neornithine from Europe illuminates the origins of crown birds. Nature. 579 (7799): 397–401.
Forster, C. A., Chiappe, L.M., Krause, D. W., Sampson, S. D. (1996): The first Cretaceous bird from Madagascar. Nature. 382 (6591): 532–534.
Forster, C. A., Sampson, S. D., Chiappe, L. M., Krause, D. W. (1998a): The theropod ancestry of birds: new evidence from the Late Cretaceous of Madagascar. Science 279:1915–1919.
Forster, C. A., Sampson, S. D., Chiappe, L. M., Krause, D. W. (1998b): Genus correction. Science. 280 (5361): 179.
Foth, C., Rauhut, O. W. M. (2017): Re-evaluation of the Haarlem Archaeopteryx and the radiation of maniraptoran theropod dinosaurs. BMC Evolutionary Biology. 17 (1): 236.
Gauthier, J. A. (1986): Saurischian monophyly and the origin of birds. Memoirs of the California Academy of Sciences. 8: 1–55.
Gauthier, J., de Queiroz, K. (2001): Feathered dinosaurs, flying dinosaurs, crown dinosaurs, and the name Aves. Pp. 7-41 in New perspectives on the origin and early evolution of birds: proceedings of the International Symposium in Honor of John H. Ostrom (J. A. Gauthier and L. F. Gall, eds.). Peabody Museum of Natural History, Yale University, New Haven, Connecticut, U.S.A.
Gauthier, J., Gall, L. F. (2001): New perspectives on the origin and early evolution of birds. New Haven: Peabody Museum of Natural History, Yale University.
Gish, D. (1995): Evolution, the Fossils Still Say NO! San Diego, Calif.: Creation-Life.
Hartman, S., Mortimer, M., Wahl, W. R., Lomax, D. R., Lippincott, J., Lovelace, D. M. (2019): A new paravian dinosaur from the Late Jurassic of North America supports a late acquisition of avian flight. PeerJ. 7: e7247.
Havstad, J. C., Smith, N. A. (2019): Fossils with feathers and philosophy of science. Systematic Biology 68, 840–851.
Heilmann, G. (1926): The origin of birds. London: H. F. & G. Witherby.
Holtz, T. R. (2007): Dinosaurs: The Most Complete, Up-to-Date Encyclopedia for Dinosaur Lovers of All Ages
Hopp, T. P., Orsen, M. J. (2004): Dinosaur Brooding Behavior and the Origin of Flight Feathers (PDF). In Currie, P. J., Koppelhus, E. B., Shugar, M. A., Wright, J. L. (eds.). Feathered dragons: studies on the transition from dinosaurs to birds. Bloomington: Indiana University Press. pp. 234−250.
Hou, L., Chiappe, L. M., Zhang, F., Chuong, C.-M, (2004): New Early Cretaceous fossil from China documents a novel trophic specialization for Mesozoic birds. Naturwissenschaften. 91 (1): 22–25.
Hou, L., Liu, Z. (1984): A new fossil bird from Lower Cretaceous of Gansu and early evolution of birds. Sci. Sin. Ser. B. 27:1296−1302.
Hou L., Martin L., Zhou Z. and Feduccia A., (1999): Archaeopteryx to opposite birds – missing link from the Mesozoic of China. Vertebrata PalAsiatica. 37(2), 88–95.
Hou, L., Zhou, Z., Gu, Y., Zhang, H. (1995): Confuciusornis sanctus, a new Late Jurassic sauriurine bird from China. In: Chinese Science Bulletin. 40, Nr. 18, S. 1545–1551.
Houston, David C., Copsey, J. A. (1994): Bone digestion and intestinal morphology of the Bearded Vulture. The Journal of Raptor Research. 28 (2): 73–78.
Hu, D., Hou, L., Zhang, L., Xu, X. (2009): A pre-Archaeopteryx troodontid theropod from China with long feathers on the metatarsus. In: Nature. Bd. 461, Nr. 7264, S. 640–643
Huxley, T. H. (1868): On the animals which are most nearly intermediate between birds and the reptiles. Ann Mag Nat Hist 2: 66–75.
Huxley, T. H. (1870): Further evidence of the affinity between the dinosaurian reptiles and birds. Q J Geol Soc 26: 12–31.
Ji, Q., Currie, P.J., Norell, M.A., Ji, S. (1998): Two feathered dinosaurs from northeastern China. Nature. 393 (6687): 753–761.
Ji, Q., Ji, S. (1996): On the discovery of the earliest bird fossil in China (Sinosauropteryx gen. nov.) and the origin of birds. Chinese Geology. 10 (233): 30–33.
Ji, Q., Ji, S. (1997): Protarchaeopterygid bird (Protarchaeopteryx gen. nov.) – fossil remains of archaeopterygids from China. Chinese Geology, 238: 38–41.
Knoll, F. et al. (2018): A diminutive perinate European Enantiornithes reveals an asynchronous ossification pattern in early birds. Nature Communicationd 9.: 937
Kuhl, H., Frankl-Vilches, C., Bakker, A., Mayr, G., Nikolaus, G., Boerno, S. T., Klages, S., Timmermann, B., Gahr, M. (2020): An unbiased molecular approach using 3’UTRs resolves the avian family-level tree of life. Molecular Biology and Evolution. 38 (1): 108–127.
Lee, M. S. Y., Cau, A., Naish, D., Dyke, G.J. (2014): Morphological Clocks in Paleontology, and a Mid-Cretaceous Origin of Crown Aves. Systematic Biology. Oxford Journals. 63 (1): 442–449.
Lee, M. S. Y., Worthy, T. H. (2011): Likelihood reinstates Archaeopteryx as a primitive bird. Biology Letters. 8 (2): 299–303.
Lee, Y.-N., Barsbold, R., Currie, P. J., Kobayashi, Y., Lee, H.-J., Godefroit, P., Escuillié, F., Chinzorig, T. (2014): Resolving the long-standing enigmas of a giant ornithomimosaur Deinocheirus mirificus. Nature. 515 (7526): 257–260.
Lefèvre, U., Cau, A., Cincotta, A., Hu, D., Chinsamy, A., Escuillié, F., Godefroit, P. (2017): A new Jurassic theropod from China documents a transitional step in the macrostructure of feathers. The Science of Nature. 104 (9–10): 74.
Lefèvre, U., Hu, D., Escuillié, F. O., Dyke, G., Godefroit, P. (2014): A new long-tailed basal bird from the Lower Cretaceous of north-eastern China. Biological Journal of the Linnean Society. 113 (3): 790–804.
Li, Q., Gao, K.-Q., Vinther, J., Shawkey, M. D., Clarke, J. A., d’Alba, L., Meng, Q., Briggs, D. E. G., Prum, R. O. (2010): Plumage color patterns of an extinct dinosaur (PDF). Science. 327 (5971): 1369–1372.
Lindgren, J., Sjövall, P., Carney, R. M., et al. (2015): Molecular composition and ultrastructure of Jurassic paravian feathers. Scientific Reports. 5: 13520
Longrich, N. (2006): Structure and function of hindlimb feathers in Archaeopteryx lithographica. Paleobiology. 32 (3): 417–431.
Longrich, N.R., Vinther, J., Meng, Q., Li, Q., Russell, A.P. (2012): Primitive wing feather arrangement in Archaeopteryx lithographica and Anchiornis huxleyi. Current Biology. 22 (23): 2262–2267.
Lü, J., Xu, L., Liu, Y., Zhang, X., Jia, S., Ji, Q. (2010): A new troodontid (Theropoda: Troodontidae) from the Late Cretaceous of central China, and the radiation of Asian troodontids. Acta Palaeontologica Polonica. 55 (3): 381–388.
Makovicky, P. J., Apesteguía, S., Agnolín, F. L. (2005): The earliest dromaeosaurid theropod from South America. Nature. 437 (7061): 1007–1011.
Marsh, Othniel C. (1872a): Preliminary description of Hesperornis regalis, with notices of four other new species of Cretaceous birds. The American Journal of Science and Arts 13-18: 360-365.
Marsh, O. C. (1872b): Notice of a new and remarkable fossil bird. American Journal of Science, series 3, 4(22):344.
McKellar, R. C., Chatterton, B. D. E., Wolfe, A. P., et al. (2011): A diverse assemblage of late cretaceous dinosaur and bird feathers from Canadian amber. Science, 333, 1619–1622.
Miller, C. V., Pittman, M., Wang, X., Zheng, X., Bright, J. A. (2022): Diet of Mesozoic toothed birds (Longipterygidae) inferred from quantitative analysis of extant avian diet proxies. BMC Biology. 20 (1): 101.
Morschhauser, E. M., Varricchio, D.J., Gao, C., Liu, J., Wang, Z., Cheng, X. & Meng, Q. (2009): Anatomy of the Early Cretaceous bird Rapaxavis pani, a new species from Liaoning Province, China. Journal of Vertebrate Paleontology. 29 (2): 545–554.
Neukamm, M., Bayer, A. (2023): Woher wissen wir, dass Vögel lebende Dinosaurier sind? Eine kritische Analyse kreationistischer Argumentation. https://www.ag-evolutionsbiologie.net/pdf/2023/evolution-warum-voegel-dinosaurier-sind.pdf
Norell, M. (2005): Unearthing Dragons: The Great Feathered Dinosaur Discoveries. New York: Pi.
Norell, M. A., Clark, J. M., Chiappe, L. M., Dashzeveg, D. (1995): A nesting dinosaur. Nature. 378 (6559): 774−776.
Norell, M. A., Clark, J. M., Dashzeveg, D., Barsbold, R., Chiappe, L. M., Davidson, A. R., McKenna, M. C., Altangerel, P., Novacek, M. J. (1994): A theropod dinosaur embryo and the affinities of the Flaming Cliffs Dinosaur eggs. Science. 266 (5186): 779−782.
Norell, M.A., Clark, J. M., Turner, A.H., Makovicky, P. J., Barsbold, R., Rowe, T. (2006): A new dromaeosaurid theropod from Ukhaa Tolgod (Omnogov, Mongolia). American Museum Novitates (3545): 1–51.
Norell, M.A., Xu, X (2005): Feathered dinosaurs. Annual Review of Earth and Planetary Sciences 33: 277–299.
O’Connor, J. K., Chiappe, L. M. (2011): A revision of enantiornithine (Aves: Ornithothoraces) skull morphology. Journal of Systematic Palaeontology. 9 (1): 135–157.
O’Connor, J. K., Zhou, Z., Smith, A. (2019): The evolution of the modern avian digestive system: insights from paravian fossils from the Yanliao and Jehol biotas. Palaeontology. 63 (1): 13–27.
O’Connor, J. K., Zhou, Z., Zhang, F. (2011): A reappraisal of Boluochia zhengi (Aves: Enantiornithes) and a discussion of intraclade diversity in the Jehol avifauna, China. Journal of Systematic Palaeontology. 9 (1): 51–63.
Osborn, H. F. (1924): Three new Theropoda, Protoceratops zone, central Mongolia. American Museum Novitates (144): 1−12.
Ostrom, John H. (1969): Osteology of Deinonychus antirrhopus, an unusual theropod from the Lower Cretaceous of Montana. Bulletin of the Peabody Museum of Natural History. 30.
Ostrom, J. H. (1974): Archaeopteryx and the origin of flight. Quarterly Review of Biology 49:27–47.
Ostrom, J. H. (1976): Archaeopteryx and the origin of birds. Biological Journal of the Linnean Society 8:91–182.
Ostrom, J. H. (1985): Introduction to Archaeopteryx. In Hecht, M. K. O., Ostrom, J. H., Viohl, G., Wellnhofer, P. (eds.). The Beginnings of Birds: Proceedings of the International Archaeopteryx Conference. Eichstätt: Freunde des Jura-Museums Eichstätt. pp. 9–20
Padian, K. (2004): Basal Avialae. In: David B. Weishampel, Peter Dodson, Halszka Osmólska (Hrsg.): The Dinosauria. 2nd edition. University of California Press, Berkeley CA u. a., S. 196–209.
Padian, K., Chiappe, L. M. (1998): The origin and early evolution of birds. Biological Reviews 73: 1–42.
Paul, G. S. (2016): The Princeton Fieldguide to Dinosaurs. 2nd Edition. Princeton University Press
Pei, R., Pittman, M., Goloboff, P. A., Dececchi, T. A., Habib, M. B., Kaye, T. G., Larsson, H. C.E., Norell, M. A., Brusatte, S. L., Xu, X. (2020): Potential for Powered Flight Neared by Most Close Avialan Relatives, but Few Crossed Its Thresholds. Current Biology. 30 (20): 4033–4046.e8.
Perle, A., Chiappe, L. M., Rinchen, B., Clark, J. M., Norell, M. A. (1994): Skeletal morphology of Mononykus olecranus (Theropoda, Avialae) from the late Cretaceous of Mongolia. American Museum Novitates (3105): 1−29.
Perle, A., Norell, M. A., Chiappe, L. M., Clark, J. M. (1993a): Flightless bird from the Cretaceous of Mongolia. Nature. 362: 623−626. Bibcode:1993Natur.362..623A. doi:10.1038/362623a0
Perle, A., Norell, M. A., Chiappe, L. M., Clark, J. M. (1993b): Correction: Flightless bird from the Cretaceous of Mongolia. Nature. 363 (188)
Perrichot, V., Marion, L., Neraudeau, D., et al. (2008): The early evolution of feathers: fossil evidence from Cretaceous amber of France. Proceedings of the Royal Society B: Biological Sciences, 275, 1197–1202.
Pittman, M., Xu, X. (2020): Pennaraptoran Theropod Dinosaurs Past Progress and New Frontiers. Bulletin of the American Museum of Natural History. 440 (1): 1–355.
Pickrell, J. (2018): Early birds may have been too hefty to sit on their eggs. Nature. doi:10.1038/d41586-018-03447-3.
Prothero, D. (2017): Evolution – What the Fossils say and why it matters. Second edition. New York: Columbia University Press
Prothero, D. (2022): Vertebrate Evolution. From Origins to Dinosaurs and beyond. CRC Press
Prum, R. O. (1999): Development and evolutionary origin of feathers. Journal of Experimental Zoology, 285, 291–306.
Prum, R. O. (2003): Are current critiques of the theropod origin of birds science? Rebuttal to Feduccia (2002). The Auk: Ornithological Advances, 120, 550–561.
Prum, R. O., Brush, A. H. (2003): Which came first, the feather or the bird? Scientific American, 288, 84–93.
Prum, R. O., et al. (2015): A comprehensive phylogeny of birds (Aves) using targeted next-generation DNA sequencing. Nature. 526 (7574): 569–573.
Rauhut, O. W. M., Foth, C. (2020): The origin of birds: current consensus, controversy, and the occurrence of feathers. In Foth, C., & Rauhut, O. (Eds.) The evolution of feathers. From their origin to the present (S. 27–45). Berlin: Springer Nature.
Rauhut, O. W. M., Foth, C., Tischlinger, H. (2020): Archaeopteryx und andere Urvögel aus dem Solnhofener Archipel. Archaeopteryx, 36, 4–15.
Roy, A., Miller, C. V., Pittmann, M., et al. (2020): Three-dimensionally preserved ‘Stage IIIb’ fossil down feather supports developmental modularity in feather evolution. BioRxiv, preprint, 1–5.
Sanz, J.L., Bonaparte, J.F., Lacasa, A. (1988): Unusual Early Cretaceous birds from Spain. Nature. 331 (6155): 433–435.
Sanz, J., Chiappe, L., Pérez-Moreno, B. et al. (1996): An Early Cretaceous bird from Spain and its implications for the evolution of avian flight. Nature 382, 442–445.
Sato, T., Cheng, Y.-N., Wu, X.-C., Zelenitsky, D. K., Hsiao, Y.-F. (2005): A Pair of Shelled Eggs Inside A Female Dinosaur. Science. 308 (5720): 375.
Schmitt, A. (2023): Großartige Giganten. Den letzten Geheimnissen der Dinosaurier auf der Spur. Dtv Verlagsgesellschaft.
Schweitzer, M. H., Schroeter, E. R., Czajka, C. D. (2021): Dinosaurs How We KNow What We Know. CRC Press
Senter, P. (2007): A new look at the phylogeny of Coelurosauria (Dinosauria: Theropoda). Journal of Systematic Palaeontology. 5 (4): 429–463.
Sereno, Paul (2001): Alvarezsaurids: Birds or ornithomimosaurs?. In Gauthier, Jacques, Ostrom, John H. (eds.). New Perspectives on the Origin and Early Evolution of Birds. Peabody Museum of Natural History Yale University.
Sereno, P. C., Rao, C. (1992): Early evolution of avian flight and perching: New evidence from the lower Cretaceous of China. Science, 255(5046), 845.
Shen, C., Zhao, B., Gao, C., Lü, J., Kundrát, M. (2017): A New Troodontid Dinosaur (Liaoningvenator curriei gen. et sp. nov.) from the Early Cretaceous Yixian Formation in Western Liaoning Province. Acta Geoscientica Sinica. 38 (3): 359–371.
Shipman, P. (1999): Taking wing: Archaeopteryx and the evolution of bird flight. New York: Simon & Schuster.
Smith, N. A., Chiappe, L. M., Clarke, J. A., et al. (2015): Rhetoric vs. reality: a commentary on Bird origins anew” by A. Feduccia. The Auk: Ornithological Advances, 132, 467–480.
Sorkin, B. (2021): Scansorial and aerial ability in Scansoriopterygidae and basal Oviraptorosauria. Historical Biology. 33 (12): 3202–3214.
Sues, H.-D. (2001): Palaeontology: ruffling feathers. Nature, 410, 1036–1037.
Thulborn, R. A. (1992): Nest of the dinosaur Protoceratops. Lethaia. 25 (2): 145−149.
Turner, A. H., Pol, D., Clarke, J. A., Erickson, G. M., Norell, M. (2007): A basal dromaeosaurid and size evolution preceding avian flight. Science. 317 (5843): 1378–1381
Turner, A. H., Makovicky, P. J., Norell, M. A. (2012): A review of dromeosaurid systematics and paravian phylogeny Bull. Am. Mus. Nat. Hist., 371:1-206
van der Reest, A.J., Wolfe, A.P., Currie, P.J. (2016): A densely feathered ornithomimid (Dinosauria: Theropoda) from the Upper Cretaceous Dinosaur Park Formation, Alberta, Canada. Cretaceous Research. 58: 108–117.
Varricchio, D. J., Moore, J. R., Erickson, G. M., Norell, M. A., Jackson, F. D., Borkowski, J. J. (2008): Avian paternal care had dinosaur origin. Science. 322 (5909): 1826−1828.
Walker, C. (1981): New subclass of birds from the Cretaceous of South America. In: Nature. 252: 51- 53.
Wang, M., Hu, H., Li, Z. (2015): A new small enantiornithine bird from the Jehol Biota, with implications for early evolution of avian skull morphology. Journal of Systematic Palaeontology. 14 (6): 481–497.
Wang, M., Zhou, Z.-H., O’Connor, J. K., Zelenkov, N. V. (2014): A new diverse enantiornithine family (Bohaiornithidae fam. nov.) from the Lower Cretaceous of China with information from two new species. Vertebrata PalAsiatica. 52 (1): 31–76.
Wang, M., Wang, X., Wang, Y., Zhou, Z. (2016): A new basal bird from China with implications for morphological diversity in early birds. Scientific Reports. 6: 19700.
Wang, X., Cau, A., Luo, X., Kundrát, M., Wu, W., Ju, S., Guo, Z., Liu, Y., Ji, Q. (2022): A new bohaiornithid-like bird from the Lower Cretaceous of China fills a gap in enantiornithine disparity. Journal of Paleontology. 96 (4): 961–976.
Wellnhofer, P. (2004): The Plumage of Archaeopteryx. In Currie PJ, Koppelhus EB, Shugar MA, Wright JL (eds.). Feathered Dragons. Indiana University Press. pp. 282–300.
Weishampel, D. B., Dodson, P., Osmólska, Halszka (2004): The Dinosauria, Second Edition. University of California Press.
Witmer, L. M. (2009): Palaeontology: Feathered dinosaurs in a tangle. Nature 461: 601–602.
Xing, L. et al. (2016): Mummified precocial bird wings in mid-Cretaceous Burmese amber. Nature Communications. 7 (1): 12089.
Xing, L et al. (2017): A mid-Cretaceous enantiornithine (Aves) hatchling preserved in Burmese amber with unusual plumage. Gondwana Research. 49: 264–277.
Xing, L et al. (2018): A flattened enantiornithine in mid-Cretaceous Burmese amber: morphology and preservation. Science Bulletin. 63 (4): 235–243.
Xing, L. et al. (2019a): A fully feathered enantiornithine foot and wing fragment preserved in mid-Cretaceous Burmese amber. Scientific Reports. 9 (1): 927.
Xing, L. et al. (2019b): A New Enantiornithine Bird with Unusual Pedal Proportions Found in Amber. Current Biology. 29 (14): 2396–2401.e2.
Xing, L. et al. (2020). An unusually large bird wing in mid-Cretaceous Burmese amber. Cretaceous Research. 110: 104412.
Xu, L., Wang, M., Chen, R., et al. (2023): A new avialan theropod from an emerging Jurassic terrestrial fauna. Nature. 621 (7978): 336–343.
Xu, X., Norell, M. A. (2004): A new troodontid dinosaur from China with avian-like sleeping posture (PDF). Nature. 431 (7010): 838–841.
Xu, X., Sullivan, C., Pittman, M., Choiniere, J. N., Hone, D., Upchurch, P., Tan, Q., Xiao, D., Tan, L., Han, F. (2011): A monodactyl nonavian dinosaur and the complex evolution of the alvarezsauroid hand. Proceedings of the National Academy of Sciences of the United States of America. 108 (6): 2338–2342.
Xu, X., Tang, Z.-L., Wang, X. L. (1999a): A therizinosauroid dinosaur with integumentary structures from China. Nature. 339 (6734): 350–354.
Xu, X., Wang, X.-L., Wu, X.-C. (1999b): A dromaeosaurid dinosaur with a filamentous integument from the Yixian Formation of China. Nature. 401 (6750): 262–266.
Xu, X., You, H., Du, K., Han, F. (2011): An Archaeopteryx-like theropod from China and the origin of Avialae (PDF). Nature. 475 (7357): 465–470.
Xu, X, Zhao, Q., Norell, M., Sullivan, C., Hone, D., Erickson, G., Wang, X., Han, F., Guo, Y. (2009): A new feathered maniraptoran dinosaur fossil that fills a morphological gap in avian origin. Chinese Science Bulletin. 54 (3): 430–435.
Xu, X., Zhou, Z., Wang, X. (2000): The smallest known non-avian theropod dinosaur. Nature 408: 705-708.
Yalden D. W. (1984): What size was Archaeopteryx? Zoological Journal of the Linnean Society. 82 (1–2): 177–188
Yang, T.-R., Chen, Y.-H., Wiemann, J., Spiering, B., Sander, P. M. (2018): Fossil eggshell cuticle elucidates dinosaur nesting ecology. PeerJ. 6: e5144.
Yang, T.-R., Wiemann, J., Xu, L., Cheng, Y.-N., Wu, X.-C., Sander, P. M. (2019): Reconstruction of oviraptorid clutches illuminates their unique nesting biology. Acta Palaeontologica Polonica. 466: 581−596.
Yonezawa, T., et al. (2017): Phylogenomics and Morphology of Extinct Paleognaths Reveal the Origin and Evolution of the Ratites. Current Biology. 27 (1): 68–77.
Zhang, F., Zhou, Z. (2000): A Primitive Enantiornithine Bird and the Origin of Feathers. Science. 290 (5498): 1955–1959.
Zhang, F., Zhou, Z., Hou, L., Gu, G. (2001). Early diversification of birds: Evidence from a new opposite bird. Chinese Science Bulletin. 46 (11): 945–949.
Zhang, F., Zhou, Z., Xu, X., Wang, X., Sullivan, C. (2008): A bizarre Jurassic maniraptoran from China with elongate ribbon-like feathers. Nature. 455 (7216): 1105–1108.
Zheng, X., Wang, X., O’Connor, J., Zhou, Z. (2012): Insight into the early evolution of the avian sternum from juvenile enantiornithines. Nature Communications. 3 (1): 1116.
Zhou, Z. (1995): Is Mononykus a Bird? In: The Auk. Bd. 112, Nr. 4, S. 958–963,
Zhou, Z. (2004): The origin and early evolution of birds: discoveries, disputes and perspectives from fossil evidence. Naturwissenschaften. 91, Nr. 10, S. 455–471
Zhou Z., Chiappe L. Zhang F., (2005): Anatomy of the Early Cretaceous bird Eoenantiornis buhleri (Aves: Enantiornithes) from China. Canadian Journal of Earth Sciences, 42 (7): 1331–1338.
Zhou, Z, Hou, L. (1998): Confuciusornis and the early evolution of birds. Vertebrata PalAsiatica. 36 (2): 136–146.
[1] https://georarities.com/2021/03/12/short-crystal-quartz-and-the-fossilized-bird/