Introduction
Hardly any other group of extinct animals fascinates young and old more than dinosaurs. The Mesozoic era is often referred to as the “age of the dinosaurs” because these amazing animals evolved in the late Triassic and dominated the landscape for over 140 million years during the rest of the Jurassic and Cretaceous periods. But even today, our planet is home to a myriad of different dinosaur species. But we’re not talking about turtles, lizards, snakes or crocodiles – we’re talking about birds. From the smallest hummingbird to the ostrich, all of the 11,000 or so living bird species described today are real dinosaurs (Chiappe 2009, Currie 2023). But why are birds real dinosaurs, but lizards and crocodiles are not? Why are genera such as Effigia, which look remarkably similar to our idea of a dinosaur (Fig. 1), not dinosaurs but rather related to crocodiles (Nesbitt et al. 2006, Nesbitt 2007, Nesbitt 2011)? And why are genera such as Silesaurus (Fig. 1), which look so similar to the genus Effigia to the layman, closer to dinosaurs, but are still not true dinosaurs (Nesbitt et al. 2010, Nesbitt 2011, Dzik 2003, Schmitt 2023)?
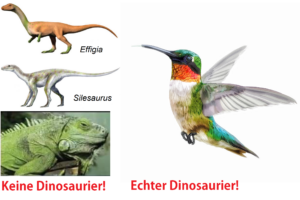
Fig.1: Effigia, Silesaurus and the green iguana are not dinosaurs. But hummingbirds are. But why?
pdf file
In the following articles, we want to explain why birds are dinosaurs and want to explore this on the basis of the fossil record, cladistics and the anatomy of these animal groups. But before we do this, we must first classify what a dinosaur is, because this is the first step in recognizing why hummingbirds are dinosaurs, but monitor lizards, crocodiles, Silesaurus and Effigia are not.
Reptiles?
Dinosaurs are generally considered to be reptiles, while birds form a separate class that at first glance has very little in common with the class of reptiles (Linneaus 1758, Fig. 2). Looking at reptiles living today, such as snakes and turtles, they have characteristics that clearly distinguish them from birds. Reptiles have scales, are usually cold-blooded, have teeth and their limbs, if they have any, are at the side of the body. Birds, on the other hand, have feathers, a toothless beak, can keep their temperature constant and their legs stand vertically under their bodies, while their arms are transformed into wings. If we consider only reptiles and birds living today, a division into two classes seems justified (cf. Modesto & Anderson 2004, Gauthier 1994).

Fig. 2: If we look at the five traditional vertebrate classes, birds and reptiles appear to have completely different characteristics that hardly suggest a close relationship.
At the same time, however, both classes have certain things in common: Even though birds have feathers, they have reptilian-like scales on their legs and toes with claws. In addition, both lay hard-shelled amniotic eggs. Most reptiles and also birds have a so-called diapsid skull structure, i.e. in addition to the eye and nasal cavities, they have two characteristic temporal fenestras on each side of the skull (Fig. 3). An upper temporal fenestra (fenestra supratemporalis, supratemporal fenestra), which usually sits on top of the skull roof, and a lower temporal fenestra (fenestra infratemporalis, infratemporal fenestra) in the side wall of the skull. In the course of the evolution of reptiles and birds, this diapside skull base type was greatly modified, but can still be traced, for example, in embryonic development. Furthermore, researchers have noticed that within the reptiles, crocodiles have more in common with birds – this can be seen, for example, in the structure of the heart, details in the skull anatomy or genetic characteristics (Benton 2007, Fastovsky & Weishampel 2021, Holtz 2007, Paul 2016, Prothero 2017, 2021, 2022, Prothero & Dott 2004, Brusatte 2019, Schmitt 2023).
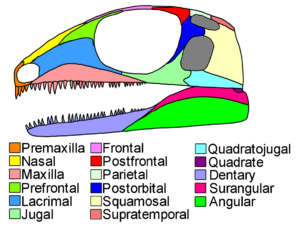
Fig. 3: Basic structure of a diapsid skull. Note the two posterior temporal fenestras marked in gray.
If we now include extinct representatives such as dinosaurs in this picture, the boundaries between dinosaurs, which are usually classified as reptiles, and birds become blurred. In biology today, care is taken to ensure that all descendants of a common ancestor are always grouped together in the systematic classification of living organisms. Scientists call this “monophyly” (Fig. 4). Such a division into monophyletic groups is based on evolutionary novelties, i.e. apomorphies that only occur in representatives of this monophyletic group. We refer to such monophyletic groups as clades. Accordingly, this type of classification of living organisms is called cladistics or phylogenetic systematics. Each lower unit is part of the next higher unit. Cladistics classifies organisms into hierarchically nested groups that are defined exclusively by evolutionary novelties (i.e. apomorphies). This creates systems of natural classes with the greatest possible objectivity, which can be translated into kinship trees (cladograms) and these in turn into phylogenetic trees.
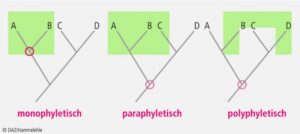
Fig. 4: Relationships: mono-, poly- and paraphyletic groups. The red circle indicates the closest branch in the phylogenetic tree that is common to all members of the group (green). Paraphyletic and polyphyletic groups do not include all individuals of a monophylum. Modern taxonomy only allows monophyletic groups.
However, the word “reptile” does not fulfill the criterion of a monophyletic group because it includes the dinosaurs but excludes the birds as their direct descendants, and is therefore a “paraphyletic group”, i.e. the term „reptile“ is imprecise and therefore strictly speaking incorrect. For this reason, a different term appears in the scientific literature, which includes all reptiles as well as birds: Sauropsida (Gauthier 1994, Fig. 5). In short, although dinosaurs had ancestors among reptiles and also close relatives such as crocodiles, even the first and most primitive dinosaurs were much more similar to birds than to classical reptiles.
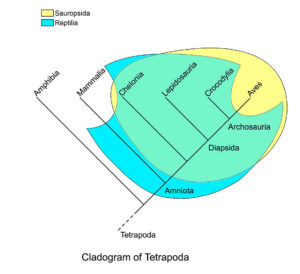
Fig. 5: Cladogram of the Tetrapoda, with Sauropsida and the class Reptilia marked. The two largely overlap, but the traditional clade Reptilia includes the earliest amniotes as well as the mammal-like reptiles, but not the birds. Sauropsida includes the birds and the recent reptile groups (turtles, lepidosaurs [= lizards and snakes] and crocodiles, as well as their common ancestors), but not the synapsids (= “mammal-like reptiles”), which are among the ancestors of modern mammals. According to this definition, the Sauropsida forms a monophyletic clade.
Archosauria
The Sauropsida are divided into several clades, the two largest of which are the Archosauromorpha and the Lepidosauromorpha (Ezcurra 2016, Martinelli et al. 2017, Butler et al. 2014, Gauthier et al. 1988, Nesbitt 2011; Fig. 6). The latter unite most classic reptiles: above all snakes and lizards, but also extinct groups, e.g. the plesiosaurs. The Archosauromorpha can be further subdivided into the Archisauriformes, in which the Archosauria are nested (Gauthier 1994, Senter 2005, Fastovsky & Weishampel 2021, Nesbitt 2011, Ezcurra 2016). The Archosauria then also include crocodiles, birds, pterosaurs and dinosaurs. This detailed bifurcation of the different clades seems confusing to the layman, but is defined by the respective apomorphies that the corresponding clades exhibit. This graded hierarchy of apomorphies is one of the best evidences of evolutionary change, where evolutionary novelties define new groups. Especially for some original representatives within some clades, it is not always easy to distinguish to which clade they exactly belong. Unfortunately, we will have no choice but to use this phylogenetic tree as a guide to finally arrive at the dinosaurs.
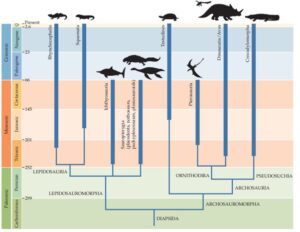
Fig. 6: Phylogenetic tree of the Diapsida a spart of the Sauropsida, which unites the two main branches of the Lepidosauromorpha and Archosauromorpha.
What features define the archosaur clade? One common feature of all archosaurs is their skull structure (Fig. 7). Based on the diapsid skull type with the two temporal fenestras, the antorbital fenestra is located in front of the eye socket. In modern birds and crocodiles, however, there have been major variations with strong specialization. Although crocodiles have clearly pronounced temporal fenestras, their antorbital fenestra is closed (Fig. 8). In birds, the antorbital fenestra is only indistinctly delimited from the eye socket and the temporal fenestras are small and also fused with the eye socket (Fig. 9). The antorbital fenestra reduced the weight of the skull, which also allowed it to grow in size. The lower jaw also originally had a fenestra, known as the mandibular fenestra, which is still present in crocodilians (Fig. 7 and 8). The attachment of the teeth to the jawbone is thecodontic, i.e. the teeth each sit in their own tooth socket (alveolus), where they are connected to the bone by connective tissue, and have no multi-part tooth roots (Fig. 7). This feature has been lost in birds due to the development of the toothless beak, but is found, for example, in some extinct bird species such as Ichthyornis.
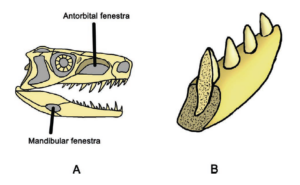
Fig. 7: Some synapomorphies of the Archosauria. A. They have two additional openings in the skull: the antorbital fenestra and the mandibular fenestra. B. The attachment of the teeth in the jawbone is thecodont, i.e. the teeth each sit in their own tooth socket (alveolus), where they are connected to the bone by connective tissue, and have no multi-part tooth roots. In contrast, the teeth of lepidosaurs sit in a groove on the inner edge of the jaw.

Fig. 8: Skull of a crocodile. The two temporal fenestras are visible, while the antorbital fenestra is secondarily regressed. The mandibular fenestra in the lower jaw is recognizable.
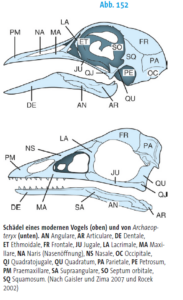
Fig. 9: Skull of a modern bird (top) compared to Archaeopteryx. The fusion of the antorbital fenestra with the eye socket is striking (both openings are separated by the lacrimal (LA).
Archosaurs also have a fourth trochanter on the femur. The fourth trochanter is a bump on the posterior-medial side of the middle of the femoral shaft that serves as a muscle attachment, mainly for the caudofemoralis longus muscle, the main retractor tail muscle that pulls the femur posteriorly, and provides a large attachment site for muscles on the femur (Nesbitt 2011, Fastovsky & Weishampel 2021, Holtz 2007, Paul 2016, Schmitt 2023; Fig. 10). Unlike lepidosaurs, such as snakes, archosaurs lack the Jacobson’s organ, an olfactory organ developed in many vertebrates (Poncelet & Shimeld 2020, Fig. 11). These apomorphies of archosaurs are of course also found in dinosaurs.
Fig. 10: Left: The left leg of a large duck-billed dinosaur with a red line drawn in, showing the approximate course of the caudofemoralis longus muscle, which ends at the fourth trochanter. Right: Reconstruction of the caudofemoralis longus muscle in Tyrannosaurus rex.
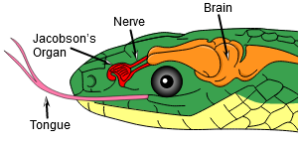
Fig. 11: Jacobson’s organ in the snake. This feature is missing in archosaurs.
Avemetatarsalia and Ornithodira
At the base of the Archosauriforma are genera such as Euparkeria or Proterosuchus (Gauthier 1986, Sookias & Butler 2013, Nesbitt 2011, Ezcurra & Butler 2015, Welman 1998), which already share some, but not all, of the apomorphies of archosaurs (Fig. 12).
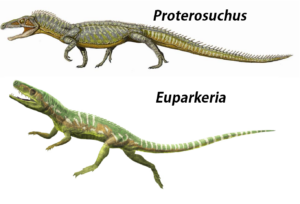
Abb. 12: Proterosuchus and Euparkeria.
The archosaurs themselves can be divided into two clades: The Crurotarsi, or Pseudosuchia, which include the crocodilians and their diverse extinct relatives, and the Avemetatarsalia, which include, among others the Dinosauromorpha and Pterosauromorpha, but also some more primitive clades (Fastovsky & Weishampel 2021, Holtz 2007, Paul 2016, Prothero 2017, 2021, 2022, Prothero & Dott 2004, Nesbitt & Norell 2007, Nesbitt 2007, 2011, Nesbitt et al. 2017, Brusatte 2019, Brusatte et al. 2010a,b, Sereno 1991, Gauthier 1986, Gauthier & de Queiroz 2001, Cau 2018, Fig. 13).
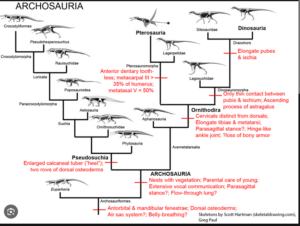
Fig. 13: Cladogram of the archosaurs with some key apomorphies
The basal Avemetatarsalia include aphanosaurs, which are at the base of the Avemetatarsalia (Nesbitt 2011, Nesbitt et al. 2017). Aphanosaurs exhibited features of both Avemetatarsalia and Crurotarsi, suggesting that they are the oldest and most ancient known genus of avemetatarsalians, at least in terms of their position in the archosaur family tree. The Dinosauromorpha and Pterosauromorpha are united as Ornithodira (Fig. 13).
A distinguishing feature between Crurotarsi and Avemetatarsalia is the structure of the ankle joint (Fastovsky & Weishampel 2021, Prothero 2017, 2021, 2022, Fig. 14). The ankle joint is the connecting joint between the lower leg and the foot. The upper tarsal bones astragalus (talus) and calcaneus (calcaneus) are involved in the ankle joint. The avemetatarsals are characterized by a large astragalus and small calcaneus. The astragalus and calcaneus are both fused to the end of the tibia and fibula. The hinge of the joint is thus located between the astragalus and calcaneus and the second row of tarsal bones. This is referred to as a mesotarsal joint, i.e. a joint in the “middle” of the tarsus and is found in all pterosaurs, dinosaurs and thus birds. This design not only enabled better mobility, but also allowed the animals to move on their toes. All dinosaurs walked on the tips of their fingers and toes and not on the palms of their hands and soles of their feet. This even applies to the heaviest giant sauropods, which still walked on their toes, but whose toe bones were squashed together into stubby columns due to their weight.
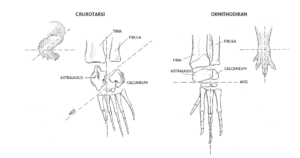
Fig. 14: Comparison of the ankles of Crurotarsi and Ornithodira (or Avemetatarsalia). See text for explanations.
For a long time, the crurotarsi were the preferred name for the crocodilian branch, as they were characterized by a pronounced ankle configuration in which the hinge of the angle runs between the calcaneus and the astragalus, with the calcaneus being larger than in the avemetatarsalia. The astragalus is only fused to the end of the tibia and the hinge runs between the calcaneus and fibula. This arrangement means that crocodilians and their relatives appear with the entire sole of the foot. However, recent analyses have shown that some groups (the phytosaurs) are more primitive than the other members of the crocodilian branch, so that the crocodilian branch is now referred to as Pseudosuchia and the phytosaurs are classified either directly outside the Pseudosuchia or, in other cases, as primitive archosauromorphs directly outside the Archosauria (Nesbitt 2011).
Dinosaurs
Within the Ornithodira, the dinosaurs, along with several other taxa, belong to the Dinosauromorpha (Benton 1985, Sereno 1991, Nesbitt 2011, Cau 2018, Gauthier 1986, Ezcurra et al. 2020). Their sister group are the Pterosauromorpha. The Dinosauromorpha have some, but not all of the apomorphies of dinosaurs. To the non-specialist, they look superficially like dinosaurs, but they are not quite dinosaurs yet. Some smaller genera such as Lagosuchus, Marasuchus and Silesaurus (Figs. 1 and 15) are among them (Romer 1971, Agnolin & Ezcurra 2019, Sereno & Arcucci 1994, Nesbitt et al. 2010, Nesbitt 2011, Dzik 2003, Schmitt 2023). Dinosaurs with their own apomorphies then emerged from these or similar forms. So here too, as with the Archosauromorpha and Archosaurs: every dinosaur belongs to the Dinosauromorpha, but not every Dinosauromorpha is a dinosaur. Which apomorphies characterize the dinosaurs?
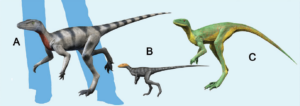
Abb. 15: some Dinosauromorpha: (A) Saltopus, (B) Marasuchus, (C) Lagerpeton.
When the very first known dinosaurs were only Megalosaurus (Buckland 1825) and Iguanodon (Mantell 1825) and a few others, Richard Owen named and diagnosed them as a group of giant extinct reptiles with a number of characteristic features (Owen 1842). As the number of new dinosaur discoveries increased rapidly in the late 1800s and early 1900s, this definition was changed. By the time Samuel Wendell Williston published Osteology of the Reptiles in 1925 (Williston 1925), dinosaurs were clearly distinguished from other reptile groups such as marine reptiles, pterosaurs and others.
In 1878, Othniel Charles Marsh recognized four groups of dinosaurs: sauropods, theropods, ornithopods and stegosaurs, groups that are still valid today. But few of these authors gave an accurate anatomical diagnosis of what constitutes a dinosaur (Prothero 2022).
In 1888, the British paleontologist Harry Govier Seeley (Seeley 1888) recognized two groups of dinosaurs: the “lizard-like” dinosaurs or Saurischia (with which he grouped the theropods and sauropods) and the “bird-like” dinosaurs or Ornithischia (which included most herbivorous dinosaurs other than the sauropods). These ideas became widely accepted over the next 130 years, and most paleontologists agreed that a fossil had to belong to one of these two groups to be a dinosaur (Fig. 16).
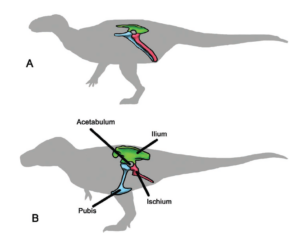
Fig. 16: The two main groups of dinosaurs: A. Bird-hipped dinosaurs (“Ornithischia”); B. Lizard-hipped dinosaurs (Saurischia)
However, Robert Bakker and Peter Galton showed in 1974 (Bakker & Galton 1974) that dinosaurs have a number of unique anatomical features that prove that they are a single natural group and did not evolve independently from different early archosaurs, the Thecodontia.
As more and more fossils were found, the differences between the two groups of dinosaurs based on their hip structure appeared to be consistent, and the individual groups (Sauropoda, Theropoda, etc.) continued to function well. The Saurischia were the dinosaurs with the lizard pelvis, where the pubic bone in the hip region pointed forward. In the Ornithischia, the “bird-hipped dinosaurs”, at least part of the pubis was shifted backwards, parallel to the rear bone of the hip region, the ischium. It is ironic that birds are derived from theropods, which had a lizard pelvis. The division into these two dinosaurs groups, which has existed since 1888, was well before the time when the relationship between dinosaurs and birds was clear. The similarity between the pelvis of Ornithischia and birds is also only superficial and not an indication of an ancestral relationship. More on this in the next articles.
However, the main suborders of dinosaurs diagnosed by Seeley did not answer the question of how the groups within the Saurischia and Ornithischia are connected. As late as the early 1970s, paleontologists were still not sure whether Saurischia and Ornithischia could be grouped together as Dinosauria. In his 1955 textbook “Evolution of the Vertebrates”, Edwin Colbert wrote that “the term covers two different orders of reptiles. Consequently, the word dinosaur is now a convenient colloquial name, but not a systematic one” (Colbert 1955).
This sad misunderstanding may have been typical of the thinking in the 1950s and 1960s when the first two editions of Colbert’s book were published (Colbert 1955, 1961), but unfortunately the text remained unchanged in the latest edition of 2001, when this idea was resoundingly debunked (Colbert et al. 2001). This was because biologists and paleontologists in the 1970s and 1980s began to use cladistics as a classification method, looking for unique evolutionary novelties that defined natural groups and moving away from so-called “wasteback” groups, which were unnatural assemblages of unrelated animals.
A number of anatomical features are now known to define dinosaurs (see Fastovsky & Weishampel 2021, Holtz 2007, Paul 2016, Prothero 2017, 2021, 2022, Prothero & Dott 2004, Bakker & Galton 1974, Brusatte et al. 2010b, Gauthier 1986, Langer et al. 2010, Nesbitt et al. 2017, Sereno 1997, 1999, Sereno et al. 1993, Brusatte 2019, Schmitt 2023, Schweitzer et al. 2021 Fig. 17).
One of the most important was defined by Jacques Gauthier in 1985 (Gauthier 1986). This concerns the acetabulum, the area of the pelvis in which the femur is inserted. In many land animals, the acetabulum is closed, but in almost all dinosaurs there is an opening (the acetabulum) that passes directly through the hip bone.
Finally, there are only three vertebrae, which are fused to the upper part of the hip bones, connecting the spine to the hind legs and forming a sacrum. Other animals have fewer or more vertebrae in their hips.
Dinosaurs have legs that are under the body (whereas in other reptiles they are on the side of the body). This position of the legs not only allows more efficient locomotion, but also enabled the gigantism of many dinosaur groups.
As mentioned, all archosaurs have a fourth trochanter, but in non-dinosaur archosaurs such as crocodilians, the fourth trochanter is rounded and symmetrical. In dinosaurs, the trochanter is clearly asymmetrical. It is assumed that this asymmetry may also have played a role in supporting their upright posture.
On the upper arm bone (humerus), dinosaurs have an elongated deltopectoral crest: Two muscles attach to this, the deltoid (the muscle on the outside of the shoulder) and the pectoralis (the chest muscles), hence the term “deltopectoral”. A longer attachment point for these muscles indicates greater strength in the forelimbs.
Dinosaurs also have a number of unique features in their skulls that distinguish them from other reptiles: they all lack the postfrontal bone, a small bone in the roof of the skull, the ectopterygoid overlaps the wing bone in the palate, the head of the temporomandibular joint (os quadratum) is exposed in lateral view and the posttemporal opening at the back of the head is smaller.
Other anatomical peculiarities of dinosaurs were a backward-oriented shoulder joint socket, asymmetrical hands with shortened fourth and fifth fingers (in many species these were missing altogether), the cnemial crest (a crest-like elevation at the upper end of the tibia), an upward-pointing projection on the astragalus and an S-shaped curved metatarsal bone. Many of these features, which at least the earlier dinosaurs from the Triassic and early Jurassic still share, have been lost or changed in later developed dinosaur species over the course of time. The anatomical blueprint of a Cretaceous dinosaur can therefore already differ greatly from that of a Triassic dinosaur.
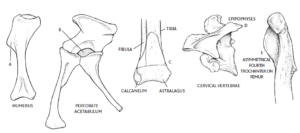
Fig. 17: Some synapomorphies of dinosaurs. a) elongated deltopectoral crest on the humerus (increases forearm strength); b) perforated acetabulum; c) fibula touching 30% of the astragalus (part of the mesotarsal bone); d) epipophyses on the cervical vertebrae (reduces rotation around the neck); and e) asymmetrical fourth trochanter on the femur.
Further characteristics of dinosaurs include the cardiovascular system and the structure of the lungs, but these will be discussed in later chapters, especially in relation to birds.
Now we’ve finally done it: Sauropsida, Archosauromorpha, Archisauriformes, Archosaurs, Avemetatarsalia, Ornithodira, Dinosauromorpha, Dinosaurs. Sorry if I have forgotten or skipped some clades in between. Thanks to anyone who has made it this far in this confusing name game. We now know what a dinosaur is and by which characteristics you can recognize one. But we haven’t really reached the dinosaurs yet, let alone the birds. When we talk about the transition from dinosaurs to birds, it is not only their place in the family tree that is interesting, but also the decisive evolutionary transitions that took place. And one thing is certain: these are very well documented and proven. But in the next episode, we will focus on the non-avian dinosaurs. Because these too not only have an astonishing diversity, but also some interesting evolutionary transitions. So if you want to know how some carnivorous dinosaurs became pure vegetarians and how small dinosaurs became large creatures such as Brachiosaurus and Argentinosaurus, take a look at the next episode on this topic.
Literatur
Agnolin, F. L., Ezcurra, M. D. (2019): The Validity of Lagosuchus Talampayensis Romer, 1971 (Archosauria, Dinosauriformes), from the Late Triassic of Argentina. Breviora. 565 (1): 1–21.
Bakker, R., Galton, P. (1974): Dinosaur Monophyly and a New Class of Vertebrates. Nature. 248. 10.1038/248168a0.
Benton, M. J. (2007): Paläontologie der Wirbeltiere. Übersetzung der 3. englischsprachigen Auflage. Verlag Dr. Friedrich Pfeil, München
Brusatte, S. L. (2019): The Rise and Fall of the Dinosaurs: A New History of a Lost World. William Morrow, NY
Brusatte, S. L., Benton, M. J., Desojo, J. B., Langer, M. C. (2010a): The higher-level phylogeny of Archosauria (Tetrapoda: Diapsida). Journal of Systematic Palaeontology. 8 (1): 3–47.
Brusatte, S. L., Nesbitt, S. J., Irmis, R. B., et al. (2010b): The origin and early radiation of dinosaurs. Earth-Science Reviews, 101, 68–100.
Buckland W. (1824): Notice on the Megalosaurus or great fossil lizard of Stonesfield. Transactions of the Geological Society of London, 1(2): 390–396.
Butler, R. J., Scheyer, T. M., Ezcurra, M. D. (2014): The Origin and Early Evolution of Sauria: Reassessing the Permian Saurian Fossil Record and the Timing of the Crocodile-Lizard Divergence. PLOS ONE. 9 (2): e89165.
Cau, A. (2018): The assembly of the avian body plan: a 160-million-year long process. Bollettino della Società Paleontologica Italiana. 57 (1): 1–25.
Chiappe, L. M. (2009): Downsized Dinosaurs: The Evolutionary Transition to Modern Birds. Evo Edu Outreach 2, 248–256
Colbert, E. H. (1955): Evolution of Vertebrates, 1st edition
Colbert, E. H. (1961): Evolution of Vertebrates, 2nd. edition
Colbert, E. H., Morales, M., Minkoff, E. C. (2001): Evolution of Vertebrates, 5th. Edition
Currie, P. J. (2023): Celebrating dinosaurs: their behaviour, evolution, growth, and physiology. Canadian Journal of Earth Sciences. 60(3): 263-293.
Dzik, J. (2003): A beaked herbivorous archosaur with dinosaur affinities from the early Late Triassic of Poland. Journal of Vertebrate Paleontology. 23 (3): 556–574.
Ezcurra, M. D. (2016): The phylogenetic relationships of basal archosauromorphs, with an emphasis on the systematics of proterosuchian archosauriforms. PeerJ. 4: e1778.
Ezcurra, M. D., Butler, R. J. (2015): Taxonomy of the proterosuchid archosauriforms (Diapsida: Archosauromorpha) from the earliest Triassic of South Africa, and implications for the early archosauriform radiation. Palaeontology. 58 (1): 141–170.
Ezcurra, M. D., Nesbitt, S. J., Bronzati, M., Dalla Vecchia, F. M., Agnolin, F. L., Benson, R. B. J., Brissón Egli, F., Cabreira, S. F., Evers, S. W., Gentil, A. R., Irmis, R. B. (2020): Enigmatic dinosaur precursors bridge the gap to the origin of Pterosauria. Nature. 588 (7838): 445–449.
Fastovsky, D. E., Weishampel, D. B. (2021): Dinosaurs: A Concise Natural History (4th ed.). Cambridge university Press, Cambridge.
Gauthier, J. A. (1986): Saurischian monophyly and the origin of birds. Memoirs of the California Academy of Sciences. 8: 1–55.
Gauthier, J. A. (1994): The diversification of the amniotes. In Prothero, D.R., Schoch, R.M. (eds.). Major Features of Vertebrate Evolution. pp. 129–159.
Gauthier, J. A., de Queiroz, K. (2001): Feathered dinosaurs, flying dinosaurs, crown dinosaurs, and the name „Aves“. pp. 7–41. In Gauthier, J. and L. F. Gall (eds.), New Perspectives on the Origin and Early Evolution of Birds: Proceedings of the International Symposium in Honor of John H. Ostrom. New Haven: Peabody Museum of Natural History, Yale University.
Gauthier, J. A., Kluge, A. G., Rowe, T. (1988): Amniote phylogeny and the importance of fossils. Cladistics. 4 (2): 105–209.
Holtz, T. R. (2007): Dinosaurs: The Most Complete, Up-to-Date Encyclopedia for Dinosaur Lovers of All Ages
Langer, M. C., Ezcurra, M. D., Bittencourt, J. S., Novas, F. E. (2010): The origin and early evolution of dinosaurs. Biological Reviews, 85, 55–110
Linnaeus, C. (1758): Systema naturae per regna tria naturae: Secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis [System of Nature through the Three Natural Kingdoms, According to classes, orders, genera, species, with characters, differences, synonyms, places] (in Latin)
Mantell G. A. (1825): Notice on the Iguanodon, a newly discovered fossil reptile, from the sandstone of Tilgate forest, in Sussex. Philosophical Transactions of the Royal Society, 115: 179–186.
Martinelli, A. G., Francischini, H., Dentzien-Dias, P. C., Soares, M. B., Schultz, C. L. (2017): The oldest archosauromorph from South America: postcranial remains from the Guadalupian (mid-Permian) Rio do Rasto Formation (Paraná Basin), southern Brazil. Historical Biology. 29 (1): 76–84.
Modesto, S. P., Anderson, J. S. (2004): The phylogenetic definition of Reptilia. Systematic Biology. 53 (5): 815–821.
Nesbitt, S. J. (2011): The early evolution of archosaurs: relationships and the origin of major clades. Bulletin of the American Museum of Natural History, 352, 1–292
Nesbitt, S. J. (2007): The anatomy of Effigia okeeffeae (Archosauria, Suchia), theropod-like convergence, and the distribution of related taxa. Bulletin of the American Museum of Natural History, 302:84
Nesbitt, S. J., Butler, R. J., Ezcurra, M. D., et al. (2017): The earliest bird-line archosaurs and the assembly of the dinosaur body plan. Nature, 544, 484–487.
Nesbitt, S. J, Norell, M. A. (2006): Extreme convergence in the body plans of an early suchian (Archosauria) and ornithomimid dinosaurs (Theropoda). Proceedings of the Royal Society B: Biological Sciences. 273 (1590): 1045–1048.
Nesbitt, S. J., Sidor, C. A., Irmis, R. B., Angielczyk, K. D., Smith, R. M. H., Tsuji, L. M. A. (2010): Ecologically distinct dinosaurian sister group shows early diversification of Ornithodira. Nature. 464 (7285): 95–98.
Owen R. (1842): Report on British fossil reptiles, part II. Report of the British Association for the Advancement of Science, 11:32–37.
Paul, G. S. (2016). The Princeton Fieldguide to Dinosaurs. 2nd Edition. Princeton University Press
Poncelet, G., Shimeld, S. M. (2020): The evolutionary origin of the vertebrate olfactory system. Open Biol. 10:200330.
Prothero, D. (2017): Evolution – What the Fossils say and why it matters. Second edition. New York: Columbia University Press
Prothero, D. (2021): The Evolving Earth. Oxford University Press
Prothero, D. (2022): Vertebrate Evolution. From Origins to Dinosaurs and beyond. CRC Press, Kapitel 11, 12, 14
Prothero, D. & Dott, (2004): Evolution of the Earth, Seventh edition. McGrawHill
Romer, A. S. (1971): The Chañares (Argentina) Triassic reptile fauna. X. Two new but incompletely known long-limbed pseudosuchians. Breviora. 378: 1–10.
Schmitt, A. (2023): Großartige Giganten. Den letzten Geheimnissen der Dinosaurier auf der Spur. Dtv Verlagsgesellschaft
Schweitzer, M. H., Schroeter, E. R., Czajka, C. D. (2021): Dinosaurs How We KNow What We Know. CRC Press
Seeley, H. G. (1888): On the classification of the Fossil Animals commonly named Dinosauria. Proceedings of the Royal Society of London. 43 (258–265): 165–171.
Senter, P. (2005): Phylogenetic taxonomy and the names of the major archosaurian (Reptilia) clades. PaleoBios. 25 (2): 1–7.
Sereno, P. C. (1991): Basal archosaurs: phylogenetic relationships and functional implications. Journal of Vertebrate Paleontology Memoir 2, 11(4, Supplement):1–53.
Sereno, P. C. (1997): The origin and evolution of dinosaurs. Annual Reviews of Earth and Planetary Sciences. 25: 435–490.
Sereno, P. C. (1999): The evolution of dinosaurs. Science. 284 (5423): 2137–2147.
Sereno, P. C., Arcucci, A. B. (1994): Dinosaurian precursors from the Middle Triassic of Argentina: Marasuchus lilloensis, gen. nov. Journal of Vertebrate Paleontology. 14 (1): 53–73.
Sereno, P.C.; Forster, C.A.; Rogers, R.R.; Monetta, A.M. (1993): Primitive dinosaur skeleton from argentina and the early evolution of Dinosauria. Nature Research. 361 (6407): 64–66.
Sookias, R. B., Butler, R. J. (2013): Euparkeriidae. Geological Society, London, Special Publications. 379 (1): 35–48.
Welman, J. (1998): The taxonomy of the South African proterosuchids (Reptilia, Archosauromorpha). Journal of Vertebrate Paleontology. 18 (2): 340–347.
Williston, S. W. (1925): Osteology of the Reptiles