Summary of parts one and two
- We humans (and most living beings) have a double set of chromosomes (= diploid). And that’s essentially true of most creatures, especially animals.
2. We humans produce, like all sexually reproducing organisms, germ cells (= gametes). These have only half a chromosome set (= haploid). The process of producing four haploid cells from a diploid cell is called meiosis (or reduction division).
3. We produce two different types of germ cells, sperm cells (small, motile) and oocytes (large, immobile). This applies to ALL animals. Other groups of organisms also produce different germ cells (always two), or they produce equivalent germ cells (isogamy), where always two merge into one zygote (but isogamy is generally uninteresting for humans and animals).
4. Living beings undergo a change in the haploid and diploid phase (= biological life cycle) in their lives. Depending on the type one phase outweighs the other phase. In humans and all animals the diploid phase dominates (= diplonts).
5. Each germ cell production coincides with a “germ cell production site”. Sperm is produced in animals in testicles, oocytes in ovaries. This is also associated with the proliferation of germ cells (internal or external fertilization, ovarian apparatus, penis, etc.).
6. If there are living things that have three or more sets of chromosomes (= polyploid), they are a special case due to disorders during meiosis. But always two types of germ cells are created.
This can justify two biological sexes = fusion of TWO haploid cells, which are BOTH of different types and EACH are formed by a certain type of “germ cell production site” (testes, ovaries). So who postulates the existence of a third (or further) sex, must prove that there is a third type of germ cells in a species that is formed by a third type of organ (which is therefore no testicle or ovary). It must be noted that each newly postulated sex must be functional (because sex fulfills a function, namely the “mixing” of the genetic material). This is also important for the remark that there are “intersex people”.
Part three deals with sex determination and development.
Sex determination – only through chromosomes?
Most depictions of human inheritance refer to sex chromosomes in the process of inheritance of the sexes. We hold on: we have 23 pairs of chromosomes in our cells (= 46 chromosomes). 22 pairs of these are called autosomal chromosomes, or called autosomes. The term autosomes simply includes all chromosomes that are not sex chromosomes. They are similar in all people, of any gender, homologous to each other. People have called a pair of sex chromosomes, also called gonosomes. These are each equipped with a sex-typical pair of chromosomes. Women have two X chromosomes (XX), men have one X and one Y chromosome (XY). We now know from germ cells that they are haploid, meaning that they only have one chromosome of a pair of chromosomes. In terms of sex chromosomes, this means that females form germ cells with one X chromosome each and males produce germ cells that have either an X or a Y chromosome. If a germ cell of the female with an X chromosome is merged with that of a male that also carries an X chromosome, then the zygote (= fertilized egg cell) has two X chromosomes (XX) and develops female. Merging a germ cell of the female (X chromosome) with a germ cell of the male, which has a Y chromosome, this results in a male (XY chromosome). Visually, this inheritance is illustrated in Fig. 1. Because the males have two different sex chromosomes, they are heterogametic. Females with XX are called homogametic.
On the Y chromosome is the SRY gene (abbreviation for “sex determining region of Y gene”). This gene codes for a transcription factor called TDF (testis determining factor). A transcription factor is defined by activating other genes (in some cases a whole series), thereby enabling the formation of important proteins. This gene is required for the expression of the male sex. We will deal later with the molecular mechanisms, as well as the embryonic development of sex development. It should be said here that the SRY gene plays an important role, but is by far not the only factor.
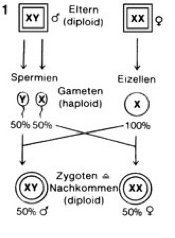
Fig. 1: Sex determination according to the XX / XY system in mammals. Source: Spectrum Biology
But there are also other forms of sexual determination via chromosomes. In birds, for example, it is the other way around. There the males have two identical sex chromosomes (ZZ), and the females have two different sex chromosomes (ZW) (see Fig. 2); Here, therefore, the males are homogametic and the females heterogametic. To delineate the XX / XY system one speaks here of a ZW / ZZ system. This form of sex determination is also found in butterflies and many snakes for example. In the ZW system of birds, a key gender-determining gene, DMRT1, is involved, which influences sex development. This occurs on the Z chromosome, but not on the W chromosome. Females have one, males two copies of this gene. Since DMRT1 is twice as common in males as females, this “overdose” leads to the development of male genitalia.
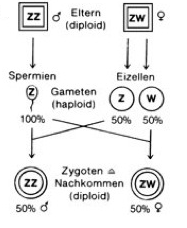
Fig. 2: Sex determination according to the ZW / ZZ system. Here the females have two different sex chromosomes. Occurrence: birds, snakes, butterflies
Another form of genetic sex determination is the XX / X0 system (Figure 3). This is a form in which one sex (usually the males) carries only one sex chromosome (X0), while the other sex (usually the female) has two (XX). This form of sexual determination can be found in many insects.
Another special form is haplodiploidy. Sex is determined by the presence of chromosomes, but there are no sex chromosomes, but diploid and haploid individuals. Such a form of sex determination can be found among the state-forming bees, wasps and ants. Here, males hatch from unfertilized eggs, which therefore have only one haploid set of chromosomes. Queens are female and have a double set of chromosomes.
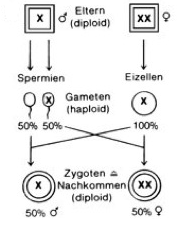
Fig. 3: Sex determination according to the X0 / XX system, which occurs in some insect groups. Source: Spectrum Biology
Also worth mentioning is the sexual determination of the fruit fly Drosophila melanogaster, the model organism of chromosome research. This species has four different pairs of chromosomes. Similar to mammals they have two sex chromosomes: females are XX, males XY. But here, not the Y chromosome, but the X chromosome carries the sex-determining genes. In the Y chromosome, however, genes are present that are necessary for sperm differentiation. The sex development in Drosophila is determined separately in each cell, namely the ratio of the X chromosomes to the autosomes (= non-sex chromosomes). Flies with only one X chromosome are male, flies with two X chromosomes female. Mutants without Y chromosome (X0) are male but sterile (they lack the genes for sperm production on the Y chromosome). On the other hand, mutants with two X chromosomes and one Y chromosome (XXY) are female, as the number of X chromosomes predominates. Since the sex is determined separately in each Drosophila cell, female embryos with two X chromosomes can originally develop male cells by the loss of an X chromosome (this also applies to birds, by the way). In insects, unlike mammals, it is not the hormonal milieu (concentration of testosterone, estrogen etc.) that determines sex development, but only the number of X chromosomes. One calls such mixed beings with male and female cells as “gynander”. Such hybrid beings can evolve through experimental control or manipulation of embryonic development that one half is male and the other is female. Such gynanders are not only found in flies, but also in butterflies or even birds (see Fig. 4). But such gynanders are not a third sex or gender, but just a mixture of male and female (i.e. two sexes) that qualitatively produce nothing new (no third germ cell type).

Fig. 4: Gynander in Birdwings (left male, right female), Drosophila (left male, right female) and Red cardinal (left female, right male).
The sexual determination does not exhaust itself on the chromosomes. Some species (and not a few of them!) determinate their sex through environmental factors, such as temperature. It occurs for example, in some lizards, fish and amphibians; in turtles and crocodiles it is the predominant, even exclusive form of sexual determination.
For example, no sea turtle or crocodile species has sex chromosomes. Their sex (and gender) depends on temperature. During a certain phase of embryonic development of these species, the outside temperature of the nest will determine whether males or females develop. In crocodiles females develop at temperatures up to 30 ° C, males only from 34 ° C. At temperatures in between, both males and females arise. In turtles, it is exactly the opposite: at low temperatures only males arise, in high females. Responsible for sex development is the enzyme aromatase, whose gene is activated by the corresponding temperatures. It converts the testosterone into estrogen. This enzyme is also found in all other vertebrates, including mammals, but is not subject to regulation by outdoor temperature. There are other forms of environmental sex determination, and some species are also known to be able to change their sex (documented in some fish species). We will discuss this in a separate article.
MANOLAKOU et al. (2006) provide a good overall survey of sex determinations in different groups of animals.
On the evolution of sex chromosomes
The question that arises naturally is why there are some species that allow their sex to be determined by chromosomes, others by environmental factors. Above all, the question arises which of these forms, at least in vertebrates, was evolutionarily in the first place.
There are several plausible theories and hypotheses. In fact, in closely related groups and species, there are both variants. The North American wood turtle (Glyptemys insculpta, family New World pond turtle Emydidae), for example, has a sex determination by chromosomes (XY), while other species of New World pond turtles, like the spotted turtle, Clemmys guttata, has its sex determined by temperature (see Jansen & Paukstis 1991a, Literman et al 2017, Montiel et al., 2017). Some lizard groups (Squamata) also have genetic as well as temperature-dependent sex determinations, such as agamas and geckos (Viets et al., 1994). JANZEN & PAUKSTIS (1991a,b) assume there could be a change between genetic and temperature-dependent sex determination in different lizard- and turtle-taxa (so related taxa evolve genetic other temperature-dependent sex determination). But some new studies (POKORNÁ KRATOCHVÍL 2009), examining the phylogenetic relationship between the taxa more closely, reveal an evolutionary transition from temperature-dependent to genetic sex determination. In this case temperature-dependent sex determination is evolutionarily plesiomorphic and if taxa develop genetic sex determination a switch back to temperature-dependent sex determination is not possible according to the authors of the study. This also explains why genetic sex determination in some groups (e.g. mammals and birds) is conserved (it always occurs).
If this was really the case, genetic sex determination would bring a selection advantage to individual groups. Sexual determination by temperature has the disadvantage that with temperature fluctuations only males or only females could arise. This could lead to extinction in species that have to reproduce sexually. In fact, due to the rising temperatures, there is the problem that from a few clutch of eggs in sea turtles almost only females hatch.
On the other hand, especially turtles and crocodiles are taxa that are much older than mammals and birds and have survived various mass extinctions of geological history. Sex determination by the temperature cannot be defined per se as “retrograde” or “disadvantageous”.
In addition, a study by QUINN et al. (2007) has shown that when male embryos of the central bearded dragon (Pogona vitticeps, homogametic chromosome set ZZ) are incubated under high temperatures, they develop into phenotypic females. So, temperature was able to change the genetic sex. It can be deduced from this experiment that temperature-dependent and genetic sex determinations can certainly co-occur, at least in some lizard species. In the ZW / ZZ system, the gender-determining genes are not on the W chromosome of the females but on the Z chromosomes. A double presence of these genes in the agama males suggests that these promote the development of the male sex, but in some species, by the influence of temperature, these males “feminize”.
POKORNÁ & KRATOCHVÍL (2009) note that although there is a co-occurrence of temperature-dependent and genetic sex determination in some species, these gonadally and phenotypically reverted individuals still possess sex chromosomes corresponding to their genotypic sex, and when crossed with non-reverted mates, they produce progeny with a skewed sex ratio. Also, the “fitness” (in the sense of reproductive success) of such “dormant” males is lower. However, Hollely et al. (2016) rightly note that sex determination in reptiles and other vertebrate animal classes, unlike mammals, is more labile and changeable. QUINN et al (2001) even note that there are different ways of developing new sex determinations in vertebrates. All possible ways of determining sex (XY, ZW, temperature dependence) may be caused by altered threshold levels of gene expression (ie how much, when, and where gene products are formed) of sex-determining genes. Under certain, slightly different, environmental conditions, labile gender-determination systems may have a selective advantage (see also SCHWANZ et al., 2013). This may also explain why some phylogenetically very old groups, such as crocodiles, have maintained a temperature-dependent sex determination. Their fitness did not suffer from this (at least in relation to the sexual determination).
This plasticity of sex determination in reptiles has been confirmed experimentally by HOLLELY et al. (2015) (also with Pogona vitticeps, but “feminized” males still behaved like males, see LI et al., 2016, and in contrast to POKORNÁ & KRATOCHVÍL 2009’s claim, they had an increased individual fitness). In amphibians (genus Xenopus), this plasticity was also reported by SARRE et al. (2011). HAYES et al. (2010) were able to induce a complete feminization of male clawed frogs (Xenopus laevis) by adding the chemical atrazine. Atrazine also reduces testicular size and sperm production in fish, reptiles and mammals (HAYES et al 2011). Thus, while sex determination in mammals and birds is essentially restricted to the chromosomes, that is, genetically fixed, it is more plastic in fish, amphibians, and reptiles (see SARRE et al., 2011).
In mammals and birds, genetic sex determination certainly has an advantage. On the one hand, unlike crocodiles and turtles, the number of offspring is relatively limited, which makes for an even ratio of the born sexes beneficial (this ratio is always 1: 1 in genetic sex determination, see Fig. 1-3). Turtles and crocodiles, however, lay many eggs, so that more offspring is produced (of which only 1% reaches sexual maturity). But there is one more important reason: Most mammal species develop in the womb, so not only in a relatively constant temperature environment but also in a female organism, with a female hormonal milieu. If this milieu had an influence on sex determination, only females would be produced (see GILBERT & EPEL 2015: 18-19). Birds, on the other hand, hatch their eggs, keeping the temperature more or less constant. Here, too, the genetic sex determination proves to be beneficial for being independent of hatching-temperature.
The most common theory of the genesis of mammalian sex chromosomes states that sex chromosomes are caused by mutations of an autosome (= non-sex chromosome). Originally, sex determination was regulated by temperature or other external environmental factors. An evolutionary model, developed by Page and Lahn (1999) suggests that the common ancestor of reptiles and mammals had a few homologous autosomal chromosomes, rather than sex chromosomes. During evolution, chromosome assembly errors occurred during meiosis, in which parts of one chromosome were mismatched by an “inversion”. An inversion is a chromosome rearrangement in which a segment of a chromosome is reversed end to end. An inversion occurs when a single chromosome undergoes breakage and rearrangement within itself. This reverses the sequence of genes on this chromosomal segment (see Fig. 5). This is how the Y-chromosome occured. Such inversions are quite common, among 10,000 births, such an inversion occurs 1.3 times. As a result of this inversion, new genes were created by further mutations, from which the SRY gene emerged. As the process progressed, the Y-chromosome lost multiple genes, thus losing homology between of the other chromosome. However, the Y-chromosome still has genes homologous to those of the X chromosome (see PAGE et al., 1984). But the area with the SRY gene of the Y chromosome is not comparable to the X chromosome, so it is typical for the Y-chromosome (see WATSON et al.1991).
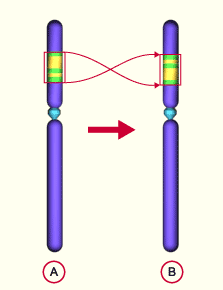
Fig. 5: Inversion. A normal chromosome, B chromosome with inversion. Source: http://www.embryology.ch/allemand/kchromaber/abweichende02.html
The females now have the following problem: They have an X-chromosome more than the males, so have a higher gene activity of certain genes. In order to prevent increased gene activity, an X chromosome is deactivated in females. This inactive X-chromosome is also called Barr body. As a consequence of X-inactivation, most of the gene products of X-linked genes have no sex differences that would otherwise be expected. Which X chromosome is inactivated, is decided by the cell and is an epigenetic process (see BARR 1949, LYON 1960, 1992, CARREL et al 1999, PAYER & LEE 2008, DISTECHE 2013).
Sex determination of one mammal group, the Monotremata (egg-laying mammals) is very fascinating. They lay, like reptiles and birds, eggs but suckle their young with milk. Today, there are very few cloacal animals, the best known is the platypus (Ornithorhynchus anatinus). A study discovered that this species does not have 2 sex chromosomes, but 10! Males have 5 XY pairs, females 5 XX pairs (ATKINSON 2004). Interestingly, studies of platypus sex chromosomes have revealed that they share similarities with mammalian X chromosomes and Z chromosomes of birds and snakes. On the other hand, there is no comparable SRY gene on the Y chromosome of the platypus as in other mammals. The 5th X chromosome, on the other hand, has the DMTR1 gene of the Z chromosomes of birds (GRÜTZNER et al., 2004a, b, VEYRUNES et al., 2008). Since the sex chromosomes of the platypuses have both similarities with the X chromosomes of other mammals, as well as the Z chromosomes of birds VEYRUNES et al. (2008) assume that the common ancestor of both groups already had sex chromosomes and these specialized in the course of evolution. The platypus sex chromosomes are not a link between the avian and mammalian chromosome but are descended from a chromosome that had homologous regions of both the ZW system of birds and the mammalian XY system. Their result means that the original mammals did not have the XY system of today’s mammals, but derived from a bird-like ZW system. Their evolution model is shown in Fig. 6:
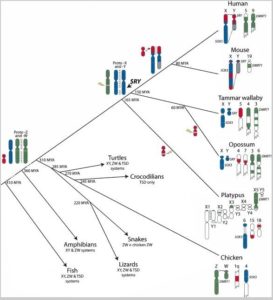
Fig. 6: Evolution of sex chromosomes according to VEYRUNES et al. 2008
Here, however, a contradictory picture emerges based on the assertion that the temperature-dependent sex determination is evolutionarily more ancestral. The family tree in Fig. 6 shows that the common ancestor of birds and mammals also includes turtles and crocodiles. Mammals, birds and “reptiles” are all grouped as “Amniota”. If the common ancestor of all amniotes already had sex chromosomes, crocodiles and turtles must have lost their sex chromosomes secondarily. This contradicts the findings of POKORNÁ & KRATOCHVÍL (2009), who postulate that once sex chromosomes are present, a return to temperature-dependent sexual determination is impossible. Most authors, however, still believe in an independent development of the sex chromosomes of birds and mammals (e.g. NAMEKWA & LEE 2009, VALLENDER & LAHN 2006).
If VEYRUNES et al. (2008) model proves to be correct, it can be assumed that the genetic sex determination of early amniotes was not so fixed, so that earlier branches of the “reptiles” could either switch between the two systems or even “return” to complete temperature-dependent sex determination.
There is no doubt that there is necessity for clarification and more research in order to solve this exciting question. The sources presented here merely provide a brief overview of existing work on sex determination and by no means claim to be complete. Should any other work not mentioned here further reinforce or refute existing hypotheses, I would be pleased to be made aware of this.
But here, too, all works have one thing in common: the reality of two biological sexes!
Müllerian’s Wolf – development of the gonads
Genetic (or environmental) sex determination is referred to as primary sex determination. It determines the fate which gonads (testes, ovaries) develop. All mammalian embryos have sexually neutral gonads, regardless of their sex chromosomes. The SRY gene on the Y chromosome provides the gonads to develop into testicles.
Secondary sexual determination denotes the development of the male and female reproductive organs. These are mainly influenced by hormones produced by gonads. Fig. 7 shows the embryonic gonads and gonadal ducts of a mammal before males and females differentiate.
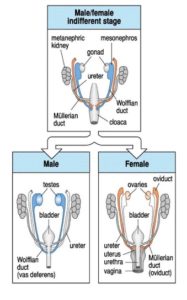
Fig. 7: Embryonic structures of the urogenital tract of a mammalian embryo before and after secondary sex determination Source: WOLPERT et al. 2007
In addition to gonads and metanephric kidney, we see the Müllerian duct (orange) and Wolfian duct, (blue).
If the gonads produce estrogen (female, XX), the Müllerian duct develops into uterine ducts, fallopian tubes (oviduct), and the upper part of the vagina (Figure 7, bottom right). If the gonads develop under the influence of the Y chromosome testicles, two important hormones are produced. The first hormone is the anti-Müllerian hormone (AMH), which degenerates the Müllerian ducts. The second hormone, testosterone, masculinizes the fetus, stimulates male genital development and suppresses breast tissue formation. Incidentally, this also explains why men have nipples: these develop before the anti-Müllerian hormone and testosterone are produced. Human embryos begin this secondary sexual differentiation from the 7th week of pregnancy. Until then, the gonads are sexually neutral and the phenotype of the embryo is female (WOLPERT et al 2007, GILBERT 2006).
In the testicular tissue, two important cell types develop: Sertoli cells and Leydig cells.
Sertoli cells serve as supportive cells of the seminiferous tubules and form the so-called blood-testis barrier, which protects the developing sperm cells from the body’s own immune system and from poisons. They also form two proteins: androgen-binding globulin (ABG), which allows testosterone to be incorporated into the sperm, and inhibin, which controls the production of sperm via the pituitary hormone FSH. The progenitor cells of the Sertoli cells also produce the anti-Müller hormone.
Another tissue in the testes, the Leydig cells, produce the testosterone. Figure 8 shows such a testicular canal with Sertoli and Leydig cells.
The epididymis, in which the sperm cells are stored and mature, develop from a part of the Wolfian duct. In addition, they produce glycoproteins (compounds of proteins and carbohydrates, in general: the ejaculate). Another area of Wolfian duct develops into the vas deferens, which fuses with the ureter and carries out the sperm.
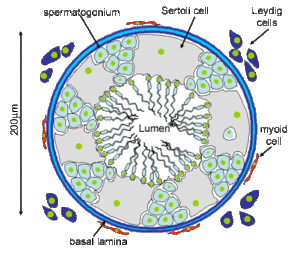
Fig. 8: Testicular tubules with Sertoli cells and primordial germ cells (spermatogonium), which are released into the lumen and pass to the vas deferens. Outside the seminiferous tubule are the Leydig cells.
In the ovaries, the oocytes (female germ cells, haploid) accumulate in the outer area and are surrounded by the body (somatic) cells (no germ cells!) of the ovary. These somatic cells develop into granulosa cells. Other cells of the ovary differentiate into theca cells, which together with granulosa cells form follicles that surround each egg and produce female sex hormones (Figure 8a). Since not enough testosterone is formed in female germ cells, the Wolfian duct degenerates.
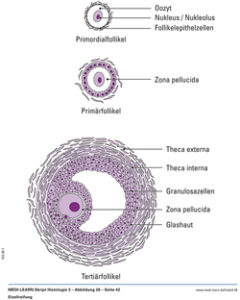
Fig. 8a: Illustration of a follicle with egg cell
Overall, the germ cell production, as well as the production of sex hormones, is under central control of the brain-hypothalamic-pituitary axis. The hypothalamus (part of the diencephalon) controls together with the pituitary (endocrine gland, just below the hypothalamus) the endocrine system of the body. The hypothalamus sends the GnRH hormone to the pituitary gland. Under the influence of GnRH, the pituitary gland releases the luteinizing hormone (LH), as well as the follicle stimulating hormone (FSH). These hormones control the maturation of germ cells and the formation of sex hormones.
Genes involved in Sex Determination
There are many genes involved in sex development. The already mentioned SRY gene in mammals is only one of many (albeit a very important one!). SRY is important for the formation of testicles. In experiments with transgenic mouse embryos, female embryos (XX) had injected the SRY gene shortly after fertilization. As a result, these injected XX genetic mice developed testes and other male reproductive organs (GILBERT 2006: p. 534). However, there are other genes involved in sex development. There is another gene, the SOX9, which is not on the sex chromosomes. Studies on genetically female mice (XX) have shown that if they have an extra copy of SOX9, they also develop testes, even if the SRY gene is missing. If the SOX9 gene is disrupted or blocked in XY males (in the presence of the SRY gene), they do not develop testes. The presence of SOX9 and SRY is necessary for testicles to develop. This should not be surprising since the SRY gene is found only in mammals, SOX9 occurs in all vertebrate taxa (GILBERT 2006: p. 535).
There are also XX males in humans, namely when the SRY gene is not on the Y chromosome but on the X chromosome. Such forms of gene replacement occur in meiosis, in which homologous chromosomes can exchange individual fragments, thereby increasing genetic variability (crossing over, Fig. 9). This can also occur in sex chromosomes (although here a crossing over is extremely rare) when the Y chromosome transfers its SRY portion to the X chromosome. When a germ cell with the “normal” X chromosome fuses with another germ cell containing the X chromosome with the SRY gene, males develop. If the Y chromosome lacks the SRY gene, XY females develop (GILBERT 2006: p. 534).
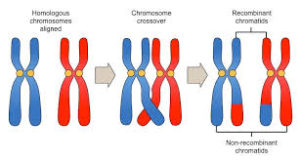
Fig. 9: Crossing over. Thus, different sections of the homologous chromosomes can be exchanged during meiosis.
Other genes that are used in the sex determination are for example the “Fibrioblast Growth Factor 9”. This gene is important for the development of Sertoli cells in the testes. The SF1 gene (Steroidogenic Factor 1) is activated either directly or indirectly by the SRY gene, which is also needed for the development of the Sertoli and Leydig cells in the testes. Together with the SOX9 gene it controls the production of the anti-Müllerian hormone and in the Leydig cells it activates the genes for the enzymes for testosterone production. The gene DAX1, on the other hand, is a testicular inhibiting gene of the X chromosome, thus involved in the formation of the ovaries. WNT4 is also a gene involved in ovarian production but is not located on the X chromosome. SRY may block the function of WNT4 so that testicles can be formed. The genes SF1 (splicing factor 1, not to be confused with steroidogenic factor 1!), WT1 (Wilms tumor genes) and LHX9 (LIM homeobox gene 9) are basically responsible for the formation of the gonadal glands that arise from the embryonic genital ridge (GILBERT 2006: p. 535 – 538, 531). Also the gene DMRT1, which plays a role in sex determination in birds, occurs in mammals and is involved in sex development (PASK, BEHRINGER & RENFREE 2001 & MATSON et al., 2010)
A schematic function of the genes and hormones described above in sex development is shown in Fig. 10 (after GILBERT 2006: p. 531).
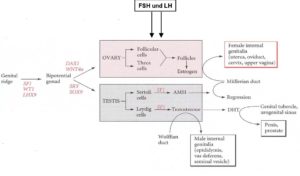
Fig. 10: Genes and hormones in the development of the sexual organs. After GILBERT 2006: p. 531
It should also be noted that these mentioned genes make up only a fraction of those who have shares in sex determination & development. Of course, it is also important to note that the right genes have to be read at the right time, in the right place and in the right quantity, as otherwise malfunctions may occur.
Dictatorship of chromosomes?
Heinz-Jürgen Voß, biologist and proponent of queer-theory and gender studies, is one of the few representatives in biological sciences, who postulates that there are more than two sexes and that this is biologically proven. In an article of the Tagesspiegel he gives the following statements:
“Until a few years ago, DNA was the secret ‘dictator’ of the cell, but is now dethroned. Today it is said that DNA does not already contain information and inform the cell about processes, rather there is a whole network of factors in the cell that interact with each other, get together and ultimately decide which actually effective product is being produced and how to read a section of the DNA, change the product, and finally fold it to make an effective product. In short, “genes” and DNA do not predict the development of an organism or, in this case, a “genital tract”. Rather, they are only one factor in the complex interplay of factors in the cell. For example, for some genes believed to be important for sex development, more than two dozen different products were found from the same gene, which perform different tasks. “Source
I agree with these statements without reservation. Already in my article on constructional morphology, I have shown that organisms are more than the sum of their genetic information. I would like to repeat some important quotes here:
Organisms cannot be reduced to “gene vehicles” (see DAWKINS 1976). Since living organisms exist in the dimensions of space and time, they cannot be deduced from the one-dimensional structure of DNA (Rose 2000, VOGEL 1991). This alone shows the above-mentioned example of muscle contraction by muscle antagonists. From the actin and myosin structure (or the genes coding for it), the muscle activity cannot be meaningfully described, only in the association of all muscles their activity explainable. Organisms are a higher and more complex union of matter (see ROSE 200: 109-111), in which new interactions and relationships arise that cannot be explained reductionistically by decomposing them into their own components. Consequently, constructional morphology criticizes the reductionism of molecular biologists.
(…)
Although genes contain important material information, but still do not have complete information for an organism’s bauplan. The genetic apparatus and its coding do not contain all the necessary “information” for the organism (Gutmann 1997). There are a variety of smaller and larger molecules, such as ions, polysaccharides and proteins, without which the DNA would not be functional. The metaphor of DNA as a book of life does not correspond to reality. DNA without a cellular environment is useless.
(…)
The idea that genes directly code for more complex structures is a misconception (LANGE 2012). WEST-EBERHARD (2003), who proves in her work that genotypic changes follow the phenotypic changes, also joins in with this idea. Embryonic development is seen as the motor of evolution and embryonic development is the result of many epigenetic regulatory processes. Changes in these processes are triggered by environmental influences.
(…)
In this respect, the concept of the germ line set up by WEISMANN (1892a, b) must also be reinterpreted. WEISMANN was able to prove that in the embryonic development of many groups of animals the primordial germ cells secrete early and unlike the somatic cells were not involved in the development of the organism. Accordingly, multicellular organisms consist of the germ cells needed for reproduction and the somatic cells that perform the bodily functions. The cell sequence from which the germ cells emerge is referred to as germline. The somatic cells branching off from the germline form the body (the soma). Because the germ line causes an uninterrupted sequence of germ cells, they are considered potentially immortal. By contrast, the body cells are subject to natural death (GRIESEMER 2002). An important consequence of this observation is that the germ cells are not affected by the somatic cells, which means that the inheritance of acquired characteristics was refuted. WEISMANN’s heredity theory forms the theoretical basis of the “Central Dogma of Molecular Biology” (CRICK 1970). Its basic statement is that no information from protein to protein or from protein to nucleic acid is possible. Watson (1965) gives a simplified version of the central dogma (DNA -> RNA -> protein), while CRICK also allowed the possibility of RNA -> DNA (e.g. in the enzyme reverse transcriptase) and DNA -> protein. As true as WEISMANN’s observation is, it is not universal because plants, fungi and some animal groups as well as unicellular organisms do not develop a germline. On the other hand, assuming a coherent and operationally closed energy-transforming organism, it is not possible for the germ cells to be independent of the rest of the organism. The germ cells are also integrated into the coherent system, not least because germ cells also need to be boosted with energy. Morphologically, this can be demonstrated by the fact that each egg cell is surrounded by nutrient cells (germ line origin) and follicular cells (somatic origin) (WOLPERT 2007). These cells supply the oocyte with RNAs, proteins, nutrients, neurotransmitters and hormones. These substances are essential for later embryonic development. The concentration of these substances as well as their transport via the nutrient and follicular cells is controlled by the organism’s neuro-hormonal network (CABEJ 2012, 2013). Nutrients such as yolk proteins are formed in the liver cells of birds and amphibians and pass through the blood to the ovaries. There, the proteins are taken up by the oocyte through phagocytosis (WOLPERT 2007). CRICK understands this one-way street of information flow reductionistically, since the DNA is elevated to the master molecule (ROSE 2000). However, the information stored in the nucleic acids is only part of the information necessary for the proteins. The only information that is in the DNA is the arrangement of the amino acids in a protein as well as appropriate regulatory sequences. From the arrangement of the amino acids alone, however, there is no blueprint of an organism possible (GUTMANN & BONIK 1981). This can be recognized solely from the fact that a protein can only develop its effect if it is folded, i.e. has a three-dimensional structure. The folding of proteins is, however, an essential part of the cellular environment (LEWONTIN 2002).
(…)
The gene regulation, i.e. when a particular gene is activated or turned off, is not coded in the DNA. JABLONKA & LAMB (2004) also showed that the inheritance of “acquired traits” does not require protein-to-DNA retranslation because the “acquired traits” do not require changes in the amino acid sequence. The reaction of organisms to changing environmental conditions does not change the amino acid sequence but the amount of certain proteins through gene regulation. Gene regulation is a complex interplay between genes, organism and environment (see CABEJ 2012, 2013, JABLONKA & LAMB 2004, GILBERT & EPEL 2009, LANGE 2012, LEWONTIN 2002). Furthermore, it could be shown that certain proteins, so-called prions, are capable of transforming other proteins with a similar or identical amino acid composition into an image of their own structure (JABLONKA & LAMB 2004, HALFMANN & LINDQUIST 2010). As a result, the flow of information from the nucleic acids (DNA, RNA) to the proteins is neither wrong nor unnecessary. However, this is not crucial for the construction of the organism, since neither in the nucleic acids nor in the proteins the information for the construction of the organisms is coded.”
Although these passages may not necessarily be understandable to some biologically not qualified reader, the key message to remember is that genes, DNA, and chromosomes are only part of the fabric of developing organisms. They play an important role in the development of organisms, but they never act independently of the environment, but are an integral part of them and can possibly be influenced by the environment (WEST-EBERHARD 2003, for example, found that environment-induced factors, e.g., environmental toxins, temperature, etc., can cause the same phenotypic changes as mutations without the DNA having to change). In part, an inheritance of acquired properties is possible because especially the egg cell in their cytoplasm stores important substances for the future development of the embryo. The storage of these substances is subject to the metabolic mechanism of the mother. Genes are not to be understood as little dictators in the Central Committee of the Chromosome House, who independently give “orders” as to how an organism has to develop. They are equal and equally dependent component in the metabolic process of the cell. In Part 2 of the Mars versus Venus series, I focused heavily on the chromosomes in germ cell formation. But of course, not only the chromosomes are divided, but the entire cell apparatus (cytoplasm, cytoskeleton, organelles, etc.).
Also, in relation to the sexual determination, we could see that at least in some species a change between genetic and temperature-dependent sexual development is possible.
But none of this suggests that the interactions between genes and environment prove the existence of more than two sexes. Of course, it would be wrong to reduce “masculinity” alone on the presence of a Y-chromosome. However, as indicated in part two, sexes are defined by their reproductive function (production of germ cells) and not by whether they are determined by the temperature or the SRY gene!
Elsewhere Voss writes in the same article:
“In genetics, for example, in model experiments on mice, about 1,000 genes have been described as potentially involved in sex development, of which just 80 have been studied, with contradictory findings. Most of these genes are usually not found on the X or Y chromosome. “
Earlier, we looked at the role of some genes in sex determinations. We have discovered that not one, but many genes are involved in sex determination and also have other functions in the organism. Here Voss really does not say anything new and he does not prove the existence of several genders.
In the next part we deal with the topic of of intersexuality.
Literature
ALBERTS, B. et. Al (2017): Molekularbiologie der Zelle. Wiley-VCH; Auflage: 6.
ATKINSON, N. (2004): Platypus has 10 sex chromosomes https://www.the-scientist.com/news-analysis/platypus-has-10-sex-chromosomes-49427
BARR ML, BERTRAM, EG: A Morphological Distinction between Neurones of the Male and Female, and the Behaviour of the Nucleolar Satellite during Accelerated Nucleoprotein Synthesis. In: Nature. 163, Nr. 4148, 1949, S. 676–7.
CARREL. L, et al. (1999): A first generation X-inactivation profile of the human X chromosome. Procl Natl Acad Sci USA. 1999, 96: 14440-14444.
DISTECHE, C. (2013): Dosage Compensation of the Sex Chromosomes. Annu Rev Genet. 2012; 46: 537–560. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3767307/
GILBERT, S. F., EPEL, D. (2009): Ecological Developmental Biology. Integrating Epigenetics, Medicine and Evolution. Sinauer Associates Inc. Publishers, Sunderland, Massachusetts USA
GILBERT, S. (2006): Developmental Biology. 8th Edition. Sinauer Associations
GRÜTZNER, F., GRAVES J.A.M., GRAVES J.A.M. (2004): A platypus’ eye view of the mammalian genome. Curr. Opin. Genet. Dev. 2004;14:642–649.
GRÜTZNER, F. et al. (2004):. In the platypus a meiotic chain of ten sex chromosomes shares genes with the bird Z and mammal X chromosomes. Nature. 2004;432:913–917.
HAYES T. et al. (2010): Atrazine induces complete feminization and chemical castration in male African clawed frogs (Xenopus laevis) Proc Natl Acad Sci U S A. 2010 Mar 9; 107(10): 4612–4617. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2842049/
HAYES T. et al. (2011): Demasculinization and feminization of male gonads by atrazine: Consistent effects across vertebrate classes J Steroid Biochem Mol Biol. 2011 Oct; 127(0): 64–73. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4303243/
HOLLELY CE, et al. (2015): Sex reversal triggers the rapid transition from genetic to temperature-dependent sex. Nature 523: 79–82 (2015). https://www.ncbi.nlm.nih.gov/pubmed/26135451
HOLLELY CE, et al. (2016): Sex Reversal in Reptiles: Reproductive Oddity or Powerful Driver of Evolutionary Change? Sex Dev 2016;10:279–287 https://www.karger.com/Article/FullText/450972
JANZEN, F.J. & PAUKSTIS (1991a): A preliminary test of the adaptive significance of environmental sex determination in reptiles. Evolution, 45: 435-440
JANZEN, F.J. & PAUKSTIS (1991b): Environmental sex determination in reptiles: Ecology, evolution and experimental design. Q. Rev. Biol., 66: 149-179
LAHN B, PAGE D (1999): Four evolutionary strata on the human X chromosome. Science. 1999, 286: 964-967.
LI, H. et al. (2016): The behavioural consequences of sex reversal in dragons Proc Biol Sci. 2016 Jun 15; 283(1832): 20160217. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4920310/
LITERMAN, R. et al.(2017): Development of sexing primers in Glyptemys insculpta and Apalone spinifera turtles uncovers an XX/XY sex-determining system in the critically-endangered bog turtle Glyptemys muhlenbergii Conservation Genet Resour December 2017, Volume 9, Issue 4, pp 651–658 https://link.springer.com/article/10.1007/s12686-017-0711-7
LYON MF (1961): Gene Action in the X-chromosome of the Mouse (Mus musculus L.) (abstract) In: Nature. 190, Nr. 4773, 1961, S. 372–3. https://www.nature.com/articles/190372a0
LYON M (1992): Some milestones in the history of X-chromosome in activation. Annu Rev Genet. 1992, 26: 17-28.
MANOLAKOU, P., et al. (2006): Molecular patterns of sex determination in the animal kingdom: a comparative study of the biology of reproduction Reproductive Biology and Endocrinology20064:59 https://rbej.biomedcentral.com/articles/10.1186/1477-7827-4-59
MATSON et al. (2010): The mammalian Doublesex homolog DMRT1 is a transcriptional gatekeeper that controls the mitosis versus meiosis decision in male germ cells Dev Cell. 2010 Oct 19; 19(4): 612–624. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2996490/
MONTIEL EE et al. (2017): Discovery of the youngest sex chromosomes reveals first case of convergent co-option of ancestral autosomes in turtles. Chromosoma. 2017 Feb;126(1):105-113. https://www.ncbi.nlm.nih.gov/pubmed/26842819
NAMEKAWA, SH & LEE JT (2009): XY and ZW: Is Meiotic Sex Chromosome Inactivation the Rule in Evolution? PLoS Genet. 2009 May; 5(5): e1000493. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2679206/
PAGE D, et al. (1984): Occurrence of a transposition from the X chromosome long arm to the Y chromosome short arm during human evolution. Nature. 1984, 311: 119-123.
PASK, BEHRINGER & RENFREE (2001): Expression of DMRT1 in the mammalian ovary and testis–from marsupials to mice. Cytogenet Genome Res. 2003;101(3-4):229-36. https://www.ncbi.nlm.nih.gov/pubmed/14684988
PAYER B, LEE JT (2008): X Chromosome Dosage Compensation: How Mammals Keep the Balance. In: Annual review of genetics. August 2008. Vol. 42:733-772 https://www.annualreviews.org/doi/10.1146/annurev.genet.42.110807.091711
POKORNÁ, M. & KRATOCHVÍL, L. (2009): Phylogeny of sex-determining mechanisms in squamate reptiles: are sex chromosomes an evolutionary trap? Zoological Journal of the Linnean Society, 2009, 156, 168–183. https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1096-3642.2008.00481.x
QUINN AE, et al. (2007): GTemperature sex reversal implies sex gene dosage in a reptile. Science 316 :41 https://www.ncbi.nlm.nih.gov/pubmed/17446395
QUINN AE, et al. (2011): Evolutionary transitions between mechanisms of sex determination in vertebrates. Biol Lett 7: 443–448. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3097877/
SARRE SD, EZAZ T, GEORGES A (2011): Transitions between sex-determining systems in reptiles and amphibians. Annu Rev Genom Hum Genet 12: 391–406. https://www.ncbi.nlm.nih.gov/pubmed/21801024
SCHWANZ LE,et al (2013): Novel evolutionary pathways of sex-determining mechanisms. J Evol Biol 26: 2544–2557 https://www.ncbi.nlm.nih.gov/pubmed/24118347
Spektrum.de: Geschlechtsbestimmung https://www.spektrum.de/lexikon/biologie-kompakt/geschlechtsbestimmung/4773
VALLENDER, E. & LAHN, B. T. (2006): Multiple independent origins of sex chromosomes in amniotes Proc Natl Acad Sci U S A. 2006 Nov 28; 103(48): 18031–18032.
VEYRUNES F et al. (2008): Bird-like sex chromosomes of platypus imply recent origin of mammal sex chromosomes. Genome Research 18: 965–973. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2413164/
WATSON JM, et al (1991): Sex chromosome evolution: Platypous gene mapping suggests that part of the human X chromosome was originally autosomal. PNAS. 1991, 88: 11256-11260.
WEST-EBERHARD, M. J. (2003): Developmental Plasticy and Evolution. Oxford University Press
WETS, B. A., EWERT,M., TALENT, L. G., NELSON, C. E. (1994): Sex-Determining mechanisms in Squamate Reptiles. Journal of Experimental Zoology 270:45-56 (1994) http://www.lacerta.de/AS/Bibliografie/BIB_1987.pdf
WOLPERT, L., JESSELL, T., LAWRENCE, P., MEYEROWITZ, E., ROBERTSON, E., SMITH, J. (2007): Principles of Development. Das Original mit Übersetzungshilfen. Third Edition. Spektrum-Verlag Berlin Heidelberg