Birds are clearly dinosaurs, as we explained in the fossil record in the last article.
In this article, we want to address the criticism of evolutionary opponents regarding the evolution of birds. Not because the pseudo-arguments of creationists have a certain plausibility or raison d’être in the scientific world, but because by debunking pseudo-science, scientific facts can be presented in an interesting didactic way and misconceptions about evolution can be cleared up in the general population, which does not necessarily sympathize with creationism. The leading representative of creationism in German-speaking countries is the evangelical study group “Wort und Wissen”, whose former managing director was Reinhard Junker. Wort und Wissen was characterized – at least under Junker’s leadership – by a certain care and superficially serious approach compared to US creationists. But their arguments are not original or really new, which is why they are representative of other creationists.
The text is part of the following work: Evolution: How do we know that birds are dinosaurs? by Martin Neukamm and Andreas Beyer[1]
Widespread convergences and conflicting phylogenetic trees
In their efforts to minimize the overwhelming phylogenetic evidence, creationists focus on supposed “anomalies” that do not fit their straw man version of the theory of evolution. They are notoriously obsessed with the problem of convergence To this end, JUNKER (2019) primarily focuses on the socalled “convergence problem.” 93 times he points out that, from an evolutionary point of view, a huge number of bird characters that also occur in non-avian theropods must have arisen convergently (independently, many times) in different lineages. According to JUNKER (2019), such findings “fit better with a creation model” than with evolution.
Indeed, evolutionary convergences and conflicting trees are quite common. However, creationists ignore several elementary facts of evolutionary and developmental biology, invalidating JUNKER’s conclusion:
They ignore “… the fact that most analyses of morphology and molecules produce congruent results” (SMITH et al. 2015, p. 473). Despite widespread convergences and uncertainty about the position of some taxa in the phylogenetic tree, “…there is a remarkable consensus on the backbone structure of the family tree of the ancestors of birds and the relative hierarchical placement of almost all major clades that constitute this tree” (RAUHUT & FOTH 2020, p. 37). In short, birds are and remain deeply nested inside Theropoda on the basis of their specific (shared derived) characters.
There are observable population-genetic mechanisms explaining incongruences (cf. PFENNINGER 2016, p. 29). One such mechanism is hybrid speciation. Restricted gene flow is often still possible for a longer period between species that split up. Depending on the genes considered, different phylogenetic trees will result. Another mechanism is “incomplete lineage sorting (Fig. 1):
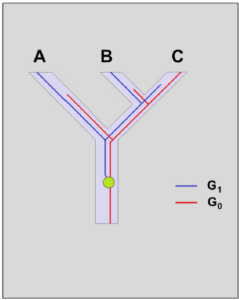
Fig. 1: Incomplete lineage sorting
Two speciation events are shown (Fig. 1): first, an ancestral species splits into two species, and later, once again, into sister species B and C. Consider the phylogenetic trajectories of the gene G, which originally occurred in a single genomic copy. Therefore, the common ancestor of A, B, and C initially possessed only allele G0. At some point, a duplication event occurred (symbolized by the green dot), and in the ancestral population, the copy G1 became fixed, and afterwards, both versions evolved independently from each other, accumulating numerous mutations. Imagine that G0 was lost in the lineage of A, whereas the ancestors of B and C retained both copies. After B and C diverged, only G1 prevailed in B, and only G0 prevailed in C. Because of the co-occurrence of G1 in species A and B, one might now think that they are sister species, although they are not. This disturbing effect is even stronger when paralogues (generated by gene duplication) arise and, much later, each lineage loses a different representative. We are dealing with an incongruence that does not reflect the actual relationships.
Some characters preferentially evolve convergently for developmental-genetic reasons (LUO et al. 2007; SHUBIN et al. 2009; MCGHEE 2011; HALL 2012; NARAMOTO et al. 2019, TOKITA et al. 2020). Convergences occur more frequently the more species are genetically similar. This phenomenon is based on development constraints. For instance, ancient homologous regulatory genes can independently be switched on and off many times in evolution to produce convergent traits: “The same forms have been produced by the repeated channeling of evolution along the same developmental trajectory” (MCGHEE 2011, p. 7).
Two things follow from all this. First, for the plausibility of the descent of birds from non-avian dinosaurs, it is irrelevant that several characters arose convergently. For this reason, individual traits are never particularly meaningful; the multitude of graded similarities corroborating birds’ deep hierarchical nesting within Theropoda is crucial. In order to create the impression of “strongly interconnected or even chaotic character distributions” (JUNKER 2019, p. 65), a crucial statistical aspect is ignored: even for a small number of considered organisms, the total number of possible trees is extremely large. For instance, if we consider 11 taxa, there are already 34 million possible unrouted trees (PENNY et al. 1982). Thus, the probability of ending up with two similar trees by chance via two independent methods, or different sets of characters, is extremely small. Moreover, even “incongruent trees” mostly show a very similar hierarchical placement of their major clades and mismatch only by some branches.
„In general, phylogenetic trees may be very incongruent and still match with an extremely high degree of statistical significance… The stunning degree of match between even the most incongruent phylogenetic trees found in the biological literature is widely unappreciated, mainly because most people (including many biologists) are unaware of the mathematics involved.“ (THEOBALD 2013).
In other words, if the characters of different species were chaotically distributed or even “freely combined (by creation)” as creationists often claim, it would be extremely unlikely to calculate even similar trees. We would have to deal with up to 34 million different trees for 10 taxa, depending on the characters we use as input. In fact, though, at worst, we end up with a few dozen alternate trees that are broadly consistent and share a very similar backbone structure, this corresponds to a measurement accuracy of 99.9999% (PENNY & HENDY 1986, p. 414)! This is a very strong phylogenetic signal indeed.
Second, considering developmental biology background knowledge, common convergences are not anomalies but rather an explicit expectation of evolutionary theory. Cladists must judge, on a case-by-case basis, how plausible convergence is. This remains uncertain without appropriate knowledge of developmental biology. However, the blanket assertion that widespread convergences speak against evolution is wrong. On the contrary, experts have been aware for decades that we can never ignore parallel developments; the more species are genetically similar, the more likely it is that the same detailed morphologies will develop in parallel (TATTERSALL 1997).
Discontinuous, “chaotic” evolution in a zigzag course
Linked to the convergence objection is the anachronistic idea that evolution must be both linear and continuous. Accordingly, JUNKER (2019) emphasizes with regard to the ability of birds to fly that, from an evolutionary perspective, there can be no talk of a “linear, step-by-step development” (p. 9). Rather, the development was “chaotic”; with regard to some characteristics, “problematic reversions” (p. 48) would have to be postulated or, in terms of evolutionary theory, a degeneration [reduction] or, as with the shoulder girdle, a kind of evolutionary zigzag course would have to be assumed, which is generally considered implausible. (JUNKER 2019, P. 62)
Apparently, only rectilinear, unidirectional changes in single, non-branching lineages (anagenetic trends) are considered for evolutionary development. However, lineage- splitting events (cladogenesis) give rise to different lines of development. Subsequently, quite separate evolutionary dynamics that unfold convey the image of a non-linear and chaotic zigzag course among lineages (see, e.g., MACFADDEN 2005). The very insistence on “continuous changes” (JUNKER 2019, p. 40) reflects obsolete ideas concerning evolution and speciation. First, developmental constraints often cause discontinuous variations (MAYNARD SMITH 1983). For instance, continuous variation of ontogenetic parameters (e.g., morphogen gradients or biomechanical forces effecting tissue interactions) can produce discontinuous changes in phenotypic traits or, in some cases, even large-scale effects, especially when threshold values are exceeded (PETERSON & MÜLLER 2016).
Second, continuous changes are not to be expected, because of the “spatial and temporal heterogeneity of the environment with its limited resources, which requires ecological segregation to avoid competition” (MAHNER 1986, p. 68). As populations establish themselves in different adaptive zones, their traits evolve at different rates—and often in different directions (FUTUYMA 2015, p. 35).
A well-studied example concerns the evolution of the horse and the splitting of its ancestral lineages in the Cenozoic (Fig. 2). As early as the 1950s, evolutionary biologist George Gaylord SIMPSON demonstrated that the phylogenetic tree of the horse does not reveal a simple, unilinear course of evolution (SIMPSON 1951). Instead, it has many side branches that have become extinct.5 Several complex lineage-splitting events occurred in horse evolution as some of the leaf-browsing genera evolved into grazers. Multiple lineages established themselves in each adaptive zone. While some grazers already had well-developed hooves, others retained their toes. Teeth, toes, and body size evolved at different tempos and modes, with high-rate variability among lineages depending on climate, vegetation, selection, and random genomic variations (MACFADDEN 2005; MIHLBACHLER et al. 2011). For more than 70 years, this “chaotic” evolution has been fully consistent with our knowledge of speciation processes. Behind this evolutionary zigzag course, a clear trend is recognizable only over many millions of years.
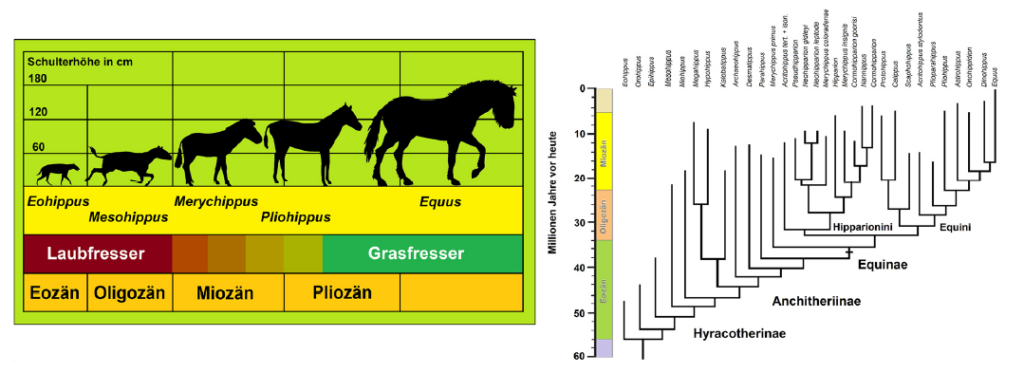
Fig. 2: Left: a simplified (linear) diagram emphasizing an anagenetic trend in horse evolution. Right diagram: how evolution proceeded considering lineage-splitting events. In the late Oligocene and Miocene, the branchings were so numerous that we cannot depict all of them. A trend toward increasing body size and reducing toes is visible only over very long periods and numerous lineages. Silhouettes of the horses: Scott HARTMAN (Hyracotherium), T. Michael KEESEY (Mesohippus), Andrew FARKE (Merychippus), Julián BAYONA (Pliohippus), and Mercedes YRAYZOZ (Equus). Source: www.phylopic.org | License: CC BY 4.0. Based on a template from www.sofatutor.com/biologie/videos/stammbaum-der-pferde. Courtesy of S. KIEFER. Phylogeny of horses according to MIHLBACHLER et al. (2011).
JUNKER quotes BRUSATTE (2017) to show that “the development of flight was chaotic” (p. 792). However, nowhere in BRUSATTE’s paper is there any mention of the need for “linear, stepwise” evolution. On the contrary, BRUSATTE refutes the view that theropods developed—or even needed—feathers and wing profiles specifically designed for flight. Instead, numerous lineages existed that possessed various potential makeshift solutions, such as skin flaps, stiffened coverts, and membranous wings to provide semi-stable wings. For example, the wings and feathers of Anchiornis were anything but tailor-made for flight (PITTMAN et al. 2022a). Nevertheless, those skin membranes’ “bridge construction” at least allowed for a gliding or weak flapping flight. On the other hand, the genus Yi, superficially resembling a bat and solely equipped with a skin membrane be- tween the fingers, only barely managed even gliding flight. The center of gravity was far behind the gliding membranes, so its flight was probably very unstable (DECECCHI et al. 2020). JUNKER’s assumption that such a chaotic developmental path, in which “dinosaurs experimented with different ways of flying” (BRUSATTE 2017, p. 792) speaks against evolution, is a poor straw man argument, born from the obsolete view that evolution must proceed linearly. The fact that JUNKER adds BRUSATTE’s metaphor of an “experimental field” as an argument for “creation,” although a chaos of different forms with many dead ends (such as Yi) fits perfectly into a non-intended natural process, is just the icing on the cake.
To sum up, morphological evolution is most commonly gradual but discontinuous, episodic, and fluctuating in direction. Most notably, evolution proceeds on multiple tracks due to numerous lineage-splitting events causing multiple lines of development in parallel. To put it another way, contrary to creationists’ premise, examples of unilinear phylogenetic paths are very rare. We can trace back phylogeny to a last common ancestor by a labyrinthine route, but no paths are straight, and all lead back by sidestepping from one event of branching speciation to another, and not by descent down a ladder of continuous change. (GOULD 2011, p. 67)
“Mismatched” mosaic forms instead of transitional forms
Creationist arguments frequently contain antiquated ideas about the nature of evolutionary transitional forms. For instance, JUNKER (2019) quotes many examples in order to show that “the mosaic of features” of the fossil in question is such that it does not fit as an evolutionary transitional form but must represent an evolutionary lineage of its own (p. 63). For instance, the bird-like theropod Rahonavis (Fig. 3) was more ‘primitive’ than Archaeopteryx with respect to some features but distinctly more birdlike with respect to others, thus not suitable as a transitional form. (p. 55)
Lineage-splitting events, followed by disparate further development, contribute to the evolution of such different mosaics of “primitive” and “advanced” characters. This finding is by no means new. For instance, even MAYR (1967, p. 465 f.) says:
„When migrating into another adaptive zone, a structure or a structural complex is under particularly strict selection pressure… As a result, this structure or complex evolves particularly fast, while others are left behind. The result is not a steady and harmonious change of all parts of the ‘type’, as idealistic biology imagines, but far more of a mosaic evolution. Each evolutionary type is a mosaic of primitive and advanced features, of general and specialized traits.“
The fact that in Archaeopteryx some traits remained more “primitive” than in Rahonavis, while others were more advanced, is not surprising against this background: the occupation of different ecological zones is accompanied by different ways of life. As a result, different selection pressures can act on the same traits in two related species. For instance, Rahonavis was an agile predator of the air with adaptations to sustained flapping flight (PITTMAN et al. 2022b). Archaeopteryx was rather a glider with lower flapping flight potential (LONGRICH et al. 2012; KSEPKA 2022), whose life took place more on the ground (ELŻANOWSKI 2002). In turn, the more primitive feature of the sickle claw accommodated the lifestyle of dromaeosaurids (FRASER 2014). Consequently, Rahonavis preserved the sickle claw.

Fig. 3: Depiction of Rahonavis (left) and Archaeopteryx (right). On the one hand, Rahonavis still has features of dromaeosaurids that proto-birds lack, such as the sickle claw on the second toe. On the other hand, in some features, it already corresponds more to the anatomy of today’s birds than the proto-bird Archaeopteryx. For example, the shoulder girdle was already quite flexible, in contrast to the fused, rigid shoulder girdle of Archaeopteryx. This feature, adapted to active flight, may have evolved in birds convergently. Thus, both mosaic forms possessed different “transitional characters” “on the way” to the birds. This shows that evolution did not proceed harmoniously along one single track for birds. Lineage-splitting events cause characters to evolve at different rates in each organism and in each lineage (heterobathmy). Left graph: artwork by James Paul BAELLO, all rights reserved. Right graphic: Author: DBCLS | Source: www.doi.org/10.7875/togopic.2020.192 | License: CC BY 4.0.
Moreover, mosaic evolution is often the result of developmental constraints or functional and genetic burdens that have their roots in the hierarchical, modular organization of traits in organisms (cf. RIEDL 2003, p. 209; FELICE & GOSWAMI 2018). Claiming that “mosaic evolution” is a “foreign body in an evolutionary scenario” (e.g., JUNKER 2019, p. 65) clearly shows a lack of knowledge of elementary principles of evolutionary biology in creationist criticism.
Given that the mode of phylogenetic development is usually mosaic evolution, what do transitional forms look like? Early anthropologists anticipated discovering fossils of human progenitors, whose features were transforming steadily into those of current humans (PROTHERO 2017, p. 135). However, due to the mosaic mode of evolution, lineages retain “primitive” features while developing “advanced” traits in parallel. The branching (speciation) process of founding independent taxa further complicates the picture. Hence, this classical expectation of the nature of transitional forms is not tenable any more (PROTHERO 2017, ibid.).
For that reason, PADIAN & ANGIELCZYK (1999) recommend shifting the focus from transitional forms to transitional features. However, the concept of transitional forms is still fruitful within the realm of cladistics if the term “transitional form” experiences a semantic shift: from a cladistic point of view, transitional forms toward birds represent extinct mosaic forms exhibiting some derived characters of crown group birds (avian synapomorphies), but not yet all of them. Additionally, those fossils still possess some ancient characters that crown group birds lack. This is the modern meaning of the term transitional form (Fig. 4).
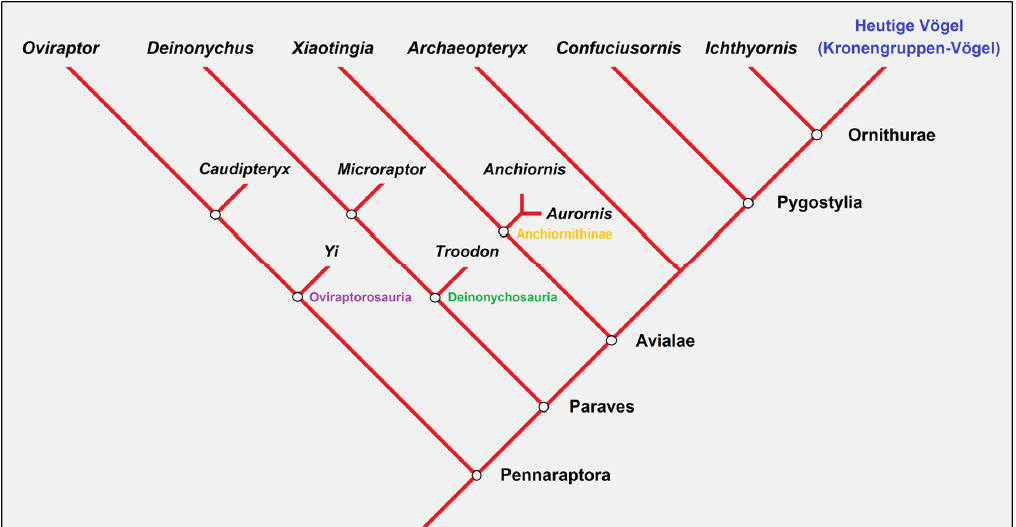
Fig. 4: Simplified cladogram of Pennaraptora, that is, the theropod clade including living birds (crown group birds). All species depicted here (and many more not shown here, which may have been direct or indirect ancestors) embody the ancestral lineage of birds. This implies they already possessed some (but not all) of the derived traits of crown group birds while still possessing quite a few non-avian theropod characteristics that living birds lack. This is exactly what is to be expected from a transitional form from a cladistic perspective. Their position in the cladogram tells us the sequence of origination of derived features in living birds. Own sketch, modified from PITTMAN & XU (2020).
Quite pointless is the attempt to dismantle the status of transitional forms by pointing out that they represent a side branch (JUNKER 2019, S. 55). Creationists often argue that many fossils exhibit progressive specialization of traits or unique distinctive characters (autapomorphies), ruling them out as direct ancestors of extant species. However, it is naive to demand that transitional forms can be strung like pearls on a necklace in procession from the Mesozoic ancestors to the modern species (crown groups) in a rectilinear ladder of change. Such thinking is “simplistic and inaccurate, reminiscent of the pre-evolutionary concepts of the ‘Great Chain of Being’ or scala naturae” (PADIAN & ANGIELCZYK 1999, p. 56). On the one hand, evolution is a branching process with a great number of dead-end branches. At least 99% of all species that have ever lived eventually became extinct (TAYLOR 2004, p. 1). Thus, fossils usually represent “dead-end” side branches, except for those few that directly lead to a crown group. However, even if we found a direct ancestor, there is no way to determine precisely how close it is to the branching point due to the incompleteness of the fossil record (PADIAN & ANGIELCZYK 1999). Thus, we must redefine the archaic meaning of the “transitional form”—a point that creationist arguments usually miss: Tree-thinking shifts the focus from looking for fossils of lineal (direct) ancestors to looking for synapomorphies that link collateral (side-branch) ancestors. (MEAD 2009, p. 311). In short: The exact position of mosaic forms, such as Archaeopteryx or Rahonavis, in the phylogenetic tree is irrelevant with respect to the integrity of the theory of evolution. Their probative force derives from the fact that mosaic forms fit into a system of graded similarities, so that we can put them in a sequence in which their morphology gradually takes on the shape of modern birds (Fig. 5).
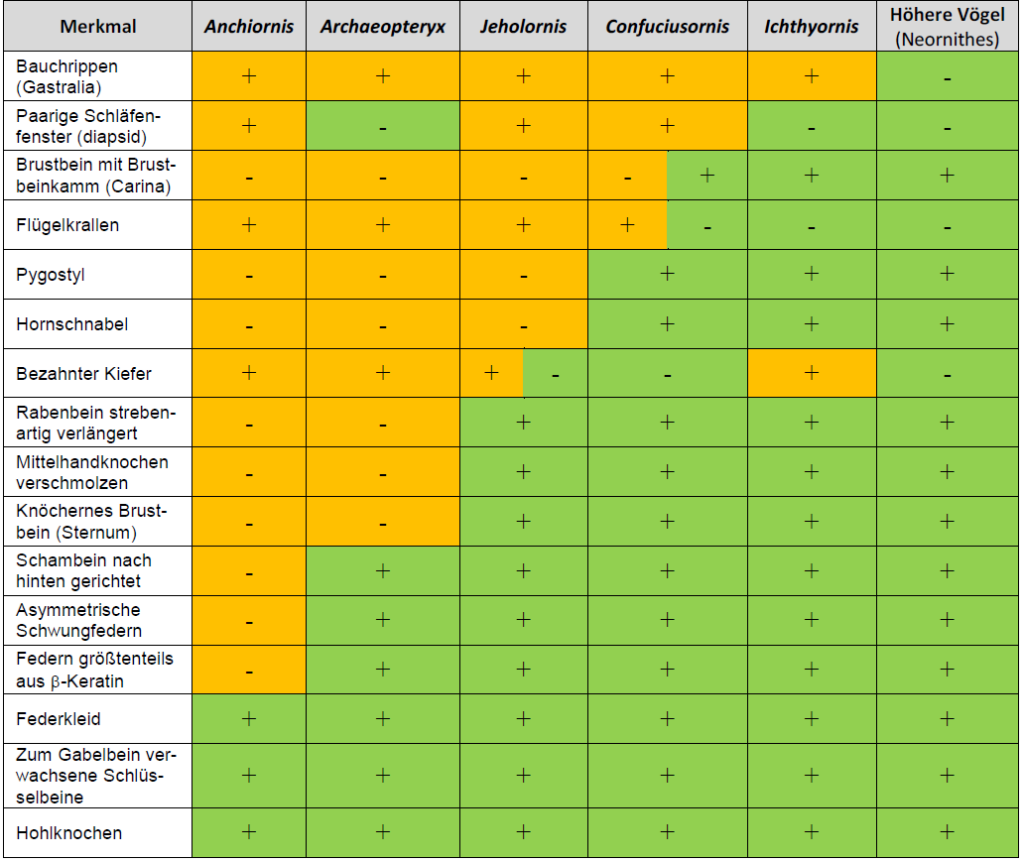
Fig. 5: Table of characters of some theropods. (+) means the feature is present, (-) the feature is absent, and (±) the feature is rudimentarily present. Orange boxes indicate the possession of primitive non-avian theropod characters, and green boxes indicate the presence of advanced avian characters. We observe that the number of avian features (shared derived traits or synapomorphies of crown group birds) gradually increases from Anchiornis via Archaeopteryx, Jeholornis, and Confuciusornis through to modern birds, as expected by evolutionary theory. The character distribution also suggests that some avian characters evolved convergently in different lineages. For example, in Archaeopteryx, independently of crown group birds, the diapsid skull changed in such a way that none of the temporal windows is clearly visible. In addition, the loss of teeth in Confuciusornis and today’s birds seems to have occurred independently.
The “discrepancy” between stratigraphy and phylogeny
Creationists often argue that the succession of species and taxa in the fossil record is not in accordance with a phylogenetic scenario. Here, we present some examples:
- Most theropod genera that have bird-like features are geologically younger than the geologically oldest birds. (JUNKER 2019)
- Cruralispennia occupies a derived position among the Enantiornithes and is not interpretable as a transitional form. Moreover, this genus is among the oldest birds after Archaeopteryx, [which means there is] a ‘stratigraphic-phylogenetic discrepancy’ (Wang et al. 2017). (JUNKER 2019, p. 52)
- Both Enantiornithes and Ornithurae appear relatively abruptly in the fossil succession in great diversity, temporally common with forms such as Confuciusornis, Jeholornis, and Sapeornis, which are classified as more primitive. (JUNKER 2019, p. 65)
- The dromaeosaurids, in turn, are placed in a broader ancestral context with birds (although they have been found in much younger strata than forms with true, flat, flight feathers). (JUNKER 2022)
Although the content of the statements is correct, they are not suitable as an objection to evolution.
In fact, the explanation for the “stratigraphic-phylogenetic discrepancy” is very simple: When more advanced bird traits evolved in some evolutionary lines, the dinosaurs with more primitive traits did not become extinct. Why should they? These differently evolved theropods coexisted for a very long time. Different evolutionary lines existed simultaneously due to species splits. When the evolutionary lineage split and one lineage developed into modern birds, the other evolutionary lineage continued to exist. (After all, “fish” still exist today, some of which became the stem tetrapods, the stem group of all terrestrial vertebrates, 380 million years ago).
To illustrate this, let’s take a look at this simplified family tree of birds (Fig. 6), with the horizons of various fossils (blue, orange and green horizontal lines).
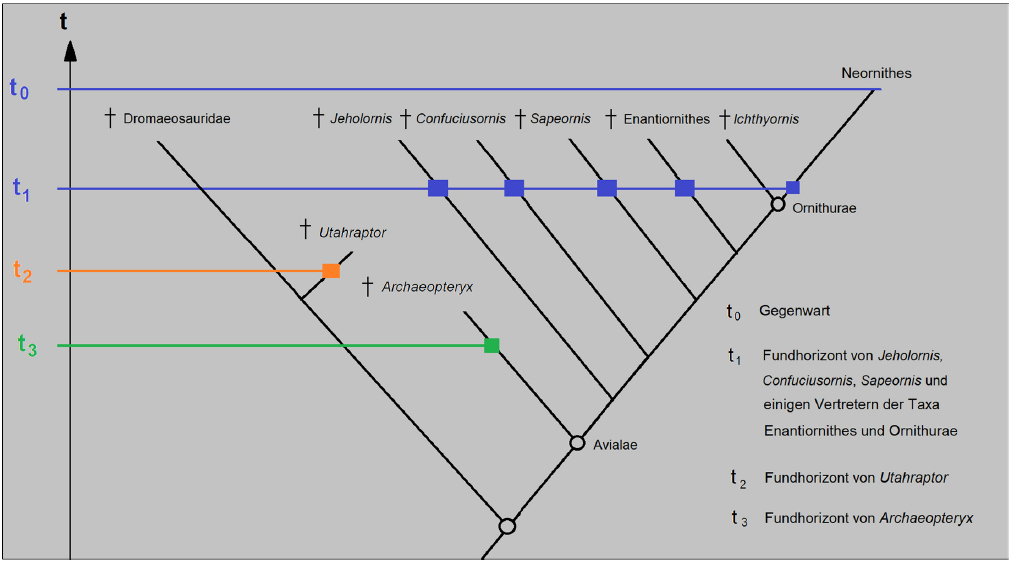
Abb. 6: Simplified phylogenetic tree of birds (Avialae) with corresponding geological strata of different fossils (blue, orange, and green horizontal lines). The extinct bird species Jeholornis, Confuciusornis, and Sapeornis are older and more primitive than the extinct Enantiornithes and the oldest representatives of Ornithurae. Nevertheless, they all coexisted over a long period, so it is not surprising that we know corresponding fossils from the same time horizon (t1). The dromaeosaurids, in turn, are more primitive than birds. However, some dromaeosaurids, such as Utahraptor, evolved later, so they are found in younger strata (t2) than, for instance, Archaeopteryx (t3).
Note that only the modern birds, the Neornithes, have survived to the present day, while the others are extinct. The phylogenetic tree also shows that the evolutionary lines that led to the extinct bird species Jeholornis, Confuciusornis and Sapeornis are older and more ancient than the likewise extinct Enantiornithes and the oldest representatives of the group Ornithurae (which includes Ichthyornis and the modern birds). This means that all these groups split off earlier in the family tree of birds. Nevertheless, these earlier splits had their own evolutionary paths and it is therefore not surprising that the genera mentioned coexisted over a long period of time and that the fossils are known from the same time horizon (t1). The dinosaur taxon of the Dromaeosauridae, on the other hand, is more ancient than that of the birds (Avialae). However, some dromaeosaurids, such as Utahraptor, evolved later, so that they are found in younger layers (t2) than Archaeopteryx, for example (t3), because the taxon of dromeosaurids also had its own evolutionary path after splitting off from the Avialae. Why not? Anyone who sees this as problematic for the theory of evolution has not understood the basics of evolution (or the principle of cladogenesis).
It should be emphasized that it is the pyhlogenetic relationships of the groups that are important, not their stratigraphic relationships (NORELL 1992).
Moreover, there are only a few rich fossil deposits in the world, which allow some sparse insights into the lives of Jurassic-Cretaceous theropods. Among these are the 150-million-year-old Solnhofen limestones from the Franconian Alb in Bavaria, where paleontologists found all (!) Archaeopteryx specimens. Since the layers of the former lagoon system formed within a few millions of years, they provide only spot checks. Thus, we cannot expect fossils to be representative of the duration of their existence in geological strata. The apparent patterns of fossil diversity are heavily distorted by uneven sampling intensity through time from geological biases that affect the temporal distribution of fossils and formations, differing preservation potential across organisms and environments, and heterogeneity in collection practice, reporting and even geopolitics. Therefore, the known fossil record is not only an incomplete sample of the total fossil record, but that incompleteness is also inconsistent through time and across space. (FLANNERY-SUTHERLAND et al. 2022).
In view of the very broadly diversified species richness of many theropod families in the Cretaceous, there must undoubtedly have been older lineages among them that go back at least to the Late Jurassic, probably even further. While individual rich deposits provide at least something like a partial overview of a certain time period, the geological time scale is less favorable elsewhere. The fauna of the early and middle Jurassic, not just the dinosaurs but all land animals, has so far been extremely poorly preserved compared to the subsequent epochs. However, such negative findings do not speak against a theropod as a bird ancestor. And the evidence no longer appears to be completely incomplete. The presence of dromaeosaurids as early as the Middle Jurassic was suggested by the discovery of individual fossil teeth, although no dromaeosaurid body fossils have been found from this period (WILLS et al. 2023).
Cladograms do not reflect the exact chronological sequence of the discovered fossils. To this end, the fossil record is only randomly sampled and unevenly distributed over millions of years. Instead, cladistics provides a comparative model of phylogenetic development by placing fossilized organisms solely on the basis of their evolutionary stage embodied in certain characteristics. In order to explore the lineage from dinosaurs to birds, it is therefore necessary to determine the degree of similarity based on characteristics that fossil creatures shared with birds or in which they differed. The fossils discovered are merely representatives of this evolution of characteristics, not necessarily the direct ancestors of the groups, and can therefore also be in the wrong place stratigraphically due to the fact that their lineages also had their own evolution.
Nevertheless, “the succession of fossils in time does not correspond to a random sequence with respect to their morphological change” (MAHNER 1986, p. 61). With the decreasing age of the layers, we find more and more bird-like theropods.
Abrupt appearance of characters and the “waiting time problem”
Creationists such as JUNKER (2019: 49; 50) mention that some features of non-bird dinosaurs appear relatively abruptly. This situation is a challenge for evolutionary mechanisms because rapid appearance is not to be expected in evolutionary theory.
This objection is meaningless because the known fossils are not at all a representative sample of extinct forms. Each instance of a fossil theropod specimen, such as the 12 known Archaeopteryx individuals, is an enormous stroke of luck. In each case, only a single specimen represents half of all dinosaur genera, and 80% of all dinosaur skeletons are only fragmentarily recorded (DODSON 1990). According to estimates, fewer than one to a few percent of species have left a fossil record (RAUP 1994). Due to the incompleteness of the fossil record, the demand for finely staggered transitions is absurd.
Furthermore, what is meant by abrupt? When a paleontologist speaks of the “abrupt” appearance of a feature, he still means periods of several tens of thousands to millions of years, whereas creationists usually think of a lightning-like emergence out of nothing.
It is also grossly misleading to claim that, due to the waiting time problem, evolutionary mechanisms are “clearly overstrained… to produce a large diversity of forms relatively abruptly in geologically short periods of time” (p. 67/93). Why is this the case?
In essence, the “waiting time problem” is based on two premises: First, evolution must
reach a “fixed and pre-specified target” (HÖSSJER et al. 2021, p. 51). Second, finding that
target would require multiple “coordinated mutations” (ibid., p. 5). Given the required ge-
netic “fine-tuning” and the fact that back mutations eliminate potentially beneficial single
mutations again, the argument goes, novelties could not evolve in realistic time periods.
To make their argument, however, creationists start from false premises. Evolution does not have to “wait” for the occurrence of predetermined target structures or functional states. Its nature is opportunistic, it uses every change that produces some kind of fitness advantage under the existing conditions. In other words, it only ever has to “wait” until a structure with any desired function emerges.
Even if a particular function is given, there is no need for evolution to wait for pre-specified DNA or protein structures. Each functional state can be accomplished in countless and totally different ways. Take antibiotic resistance as an example: Among others, antibiotics can be rendered ineffective by novel enzymes, modified receptors, efflux pumps, or “up-regulating” an antagonistic signaling pathway. Each of these pathways, in turn, has multiple routes open to evolution. In terms of the enzymatic route, for instance, cleavage or acetylation can inactivate a drug. For each route, evolution can in turn use numerous different options. For example, the enzyme class of beta-lactamases is highly diverse. It includes protein families that have little structural similarity to each other (HUNT 2007a). Finally, each individual protein can exhibit enormous variability while maintaining its function.
The assumption that multiple coordinated mutations are necessarily required to achieve a target also proves to be false. The emergence of enzymes with completely novel properties can often be accomplished by single mutations (DE KRAKER & GERSHENZON 2011). Analogous to this finding, YONA et al. (2018) showed that ~ 60% of purely random DNA sequences containing no functional information (!) are only one mutation away from turning into active promoter sequences. A similar study demonstrated that a high percentage of randomized peptides (when attached to the end of a cytosolic protein) can serve as functional targeting signal for specific import into a certain cell organelle (TONKIN et al. 2008). This is because thousands of (cryptic) genes, signaling pathways, and co-factors imply an enormous number of candidate combinations for complex gene interactions.
Summary
Evolution deniers portray the convergence problem as much more serious than it is. Despite widespread convergence and uncertainty about the position of some taxa, there is a remarkable consensus on the backbone structure of the family tree of the ancestors of birds and the relative hierarchical placement of almost all major clades that constitute this tree. Birds still lie in a deeply nested position within Theropoda (RAUHUT & FOTH 2020, p. 37).
Contrary to creationists’ reasoning, widespread convergences are not anomalies but rather a clear expectation of evolutionary theory when developmental biological background knowledge is considered (MCGHEE 2011, p. 7).
For at least half a century, the idea that evolution must run continuously and linearly has not been compatible with contemporary knowledge of the processes of speciation and differentiation of species (MAHNER 1986, p. 68). Creationists do not seem to know that just these processes are accompanied by discontinuities, incongruities, and a zigzag course (such as reversions of traits in different lineages).
Creationists place antiquated expectations on the nature of evolutionary transitional forms and claim to have recognized “contradictory” trait mosaics. They fail to recognize that mosaic evolution is precisely the result of lineage-splitting events (MAYR 1967, p. 465f) and genetic burdens (RIEDL 2003, p. 209). Creationists seem not to understand cladogenesis; otherwise, they would not problematize the “phylogenetic-stratigraphic discrepancy,” i.e., the chronologically later appearance of some species with more primitive features in the fossil record.
No alternative hypothesis has withstood cladistic testing; and, in fact, there have not been any specific alternative hypotheses for over 20 years. No other method of phylogenetic analysis has been proposed and argued to supplant cladistics, which is why the field, as a whole, remains unconvinced by these objections (PADIAN & HORNER 2002).
So far, we have examined our focus on bird evolution using the fossil record and cladistics (including the baseless critiques of creationism). But that is not enough. Birds are highly specialized dinosaurs, most of whose representatives are able to fly, and all birds alive today that cannot fly have lost their ability to fly secondarily. But how did the evolution of flight come about? What anatomical requirements and changes have to occur for a feathered dinosaur to take to the skies? We will find out in the next articles.
Literature
BRUSATTE, S. L. (2017). A Mesozoic aviary. Science, 355, 792–794.
DECECCHI, T. A., ROY, A., PITTMAN, M., et al. (2020). Aerodynamics show membrane-winged theropods were a poor gliding dead-end. iScience, 23, 101574.
DE KRAKER, J.-W., & GERSHENZON, J. (2011). From amino acid to glucosinolate biosynthesis: protein sequence changes in the evolution of methylthioalkylmalate synthase in Arabidopsis. The Plant Cell, 23, 38–53.
DODSON, P. (1990). Counting dinosaurs: how many kinds were there? Proceedings of the National Academy of Sciences, 87, 7608–7612.
ELŻANOWSKI, A. (2002). Biology of basal birds and the origin of avian flight. In ZHOU, Z., & ZHANG, F. (Eds.) Proceedings of the 5thSymposium of the Society of Avian Paleontology and Evolution (S. 211–226). Beijing: Science Press.
FELICE, R. N., & GOSWAMI, A. (2018). Developmental origins of mosaic evolution in the avian cranium. PNAS, 115, 555–560.
FLANNERY-SUTHERLAND, J. T., SILVESTRO, D., & BENTON, M. J. (2022). Global diversity dynamics in the fossil record are regionally heterogeneous. Nature Communications, 13, 2751.
FRASER, G. (2014). “Bizarre structures” point to dromaeosaurs as parasites and a new theory for the origin of avian flight. The Journal of Paleontological Sciences, 6, 1–27.
FUTUYMA, D. J. (2015). Can modern evolutionary theory explain macroevolution? In SERRELLI, E., & GONTIER, N. (Eds.) Macroevolution. Explanation, interpretation, and evidence (S. 29–85). Heidelberg: Springer.
GOULD, S. J. (2002). Illusion Fortschritt. Die vielfältigen Wege der Evolution, 2. Auflage. Frankfurt: S. Fischer Verlag.
HALL, B. K. (2012). Parallelism, deep homology, and evo-devo. Evolution & Development, 14, 29–33.
HÖSSJER, O., BECHLY, G., & GAUGER, A. (2021). On the waiting time until coordinated mutations get fixed in regulatory sequences. Journal of Theoretical Biology, 524, 110657. https://doi.org/10.1016/j.jtbi.2021.110657.
JUNKER, R. (2019). Sind Vögel Dinosaurier? Eine kritische Analyse fossiler Befunde. https://www.tinyurl.com/msw952vh (Kurzversion), https://www.tinyurl.com/4eutmru7 (Langfassung).
JUNKER, R. (2022). Flugsaurier mit Federn? Begriffsverwirrung führt zu verwirrenden Aussagen. https://www.tinyurl.com/yn7p9b6p.
KSEPKA, D. T. (2022). Evolution of birds. In SCANES, C. G., & DRIDI, S. (Eds.) Sturkie’s avian physiology, 7th Edition (S. 83–107). Cambridge: Academic Press.
LONGRICH, N. R., VINTHER, J., MENG, Q., et al. (2012). Primitive wing feather arrangement in Archaeopteryx lithographica and Anchiornis huxleyi. Current Biology, 22, 2262–2267.
LUO, Z.-X., CHEN, P., LI, G., et al. (2007). A new eutriconodont mammal and evolutionary development in early mammals. Nature, 446, 288–293.
MACFADDEN, B. J. (2005). Fossil horses – evidence for evolution. Science, 307, 1728–1730.
MAHNER, M. (1986). Kreationismus. Inhalt und Struktur antievolutionistischer Argumentation. Berlin: Pädagogisches Zentrum Berlin.
MAYNARD SMITH, J. (1983). The genetics of stasis and punctuation. Annual Review of Genetics 17, 11–25.
MAYR, E. (1967). Artbegriff und Evolution. Singhofen: Verlag Paul Parey.
MCGHEE, G. R. (2011). Convergent evolution. Limited forms most beautiful. Cambridge: The MIT Press.
MEAD, L. S. (2009). Transforming our thinking about transitional form. Evolution: Education and Outreach, 2, 310–314.
MIHLBACHLER, M. C., RIVALS, F., SOLOUNIAS, N., et al. (2011). Dietary change and evolution of horses in North America. Science, 331, 1178–1181.
NARAMOTO, S., JONES, V. A. S., TROZZI, N., et al. (2019). A conserved regulatory mechanism mediates the convergent evolution of plant shoot lateral organs. PLoS Biology, 17, e3000560.
NORELL, M. A. (1992). Taxic origin and temporal diversity: the effect of phylogeny. In NOVACEK, M. J., & WHEELER, Q. D. (Eds.) Extinction and phylogeny (S. 89–118). New York: Columbia University Press.
PADIAN, K., & ANGIELCZYK, K. D. (1999). Are there transitional forms in the fossil record? The Paleontological Society Papers, 5, 47–82.
PADIAN, K., & HORNER, J. R. (2002). Typology versus transformation in the origin of birds. Trends in Ecology & Evolution, 17, 120–124.
PENNY, D., FOULDS, L. R., & HENDY, M. D. (1982). Testing the theory of evolution by comparing phylogenetic trees constructed from five different protein sequences. Nature, 297, 197–200.
PENNY, D., & HENDY, M. D. (1986). Estimating the reliability of phylogenetic trees. Molecular Biology and Evolution, 3, 403–417.
PETERSON, T., & MÜLLER, G. B. (2016). Phenotypic novelty in EvoDevo: the distinction between continuous and discontinuous variation and its importance in evolutionary theory. Evolutionary Biology, 43, 314–335.
PFENNINGER, M. (2016). Artbegriff im Wandel der Zeit. In LOZÁN, J. L., et al. (Eds.) Warnsignal Klima: Die Biodiversität (S. 26–31). Hamburg: Verlag Wissenschaftliche Auswertungen.
PITTMAN, M., & XU, X. (2020). Pennaraptoran theropod dinosaurs. Past progress and new frontiers. Bulletin of the American Museum of Natural History, 440, 1–355.
PITTMAN, M., BELL, P. R., MILLER, C. V., et al. (2022a). Exceptional preservation and foot structure reveal ecological transitions and lifestyles of early theropod flyers. Nature Communications, 13, 7684.
PITTMAN, M., Kaye, T. G., Wang, X., et al. (2022b). Preserved soft anatomy confirms shoulder-powered upstroke of early theropodflyers, reveals enhanced early pygostylian upstroke, and explains early sternum loss. PNAS, 119, e2205476119.
PROTHERO, D. R. (2017). Evolution. What the fossils say and why it matters. 2nd edition. New York Chichester, West Sussex: Columbia University Press.
RAUHUT, O. W. M., & FOTH, C. (2020). The origin of birds: current consensus, controversy, and the occurrence of feathers. In FOTH, C., & RAUHUT, O. (Eds.) The evolution of feathers. From their origin to the present (S. 27–45). Berlin: Springer Nature.
RAUP, D. M. (1994). The role of extinction in evolution. Proceedings of the National Academy of Science, 91, 6758–6763.
RIEDL, R. (2003). Riedls Kulturgeschichte der Evolutionstheorie. Berlin: Springer.
SHUBIN, N., TABIN, C., & CARROLL, S. (2009). Deep homology and the origins of evolutionary novelty. Nature, 457, 818–823.
SIMPSON, G. G. (1951). Horses. The story of the horse family in the modern world and through sixty million years of history. New York: Oxford University Press.
SMITH, N. A., CHIAPPE, L. M., CLARKE, J. A., et al. (2015). Rhetoric vs. reality: a commentary on “Bird origins anew” by A. Feduccia. The Auk: Ornithological Advances, 132, 467– 480.
TATTERSALL, I. (1997). Puzzle Menschwerdung. Auf der Spur der menschlichen Evolution. Heidelberg: Spektrum Akademischer Verlag.
TAYLOR, P. (2004). Extinction in the history of life. Cambridge: Cambridge University Press.
THEOBALD, D. (2013). 29+ Evidences for macroevolution. Part 1: The unique universal phylogenetic tree. https://www.tinyurl.com/4kzfp5jt.
TOKITA, M., MATSUSHITA, H., & ASAKURA, Y. (2020). Developmental mechanisms underlying webbed foot morphological diversity in waterbirds. Scientific reports, 10, 8028.
TONKIN, C. J., FOTH, B. J., RALPH, S. A., et al. (2008). Evolution of malaria parasite plastid targeting sequences. PNAS, 105, 4781–4785.
WANG, X., PITTMAN, M., ZHENG, X., et al. (2017). Basal paravian functional anatomy illuminated by high-detail body outline. Nature Communications, 8, 14576.
WILLS, S.; UNDERWOOD, C. J.; BARETT, P. M. (2023). Machine learning confirms new records of maniraptoran theropods in Middle Jurassic UK microvertebrate faunas. Papers in Palaeontology. 9 (2). e1487.
YONA, A. H., ALM, E. J., & GORE, J. (2018). Random sequences rapidly evolve into de novo promoters. Nature Communications, 9, 1530.
[1] https://www.ag-evolutionsbiologie.net/html/2023/evolution-warum-voegel-dinosaurier-sind.html