In the previous parts we have either dealt with absurd “philosophical” views (Butler’s queer theory or Kutschera’s sympathies for AfD and Catholic church reactionaries) or have penetrated deep into genetics. At this point, we now deal with mating systems in the animal kingdom. Anyone who has an interest in how diverse the sex life of animals is without much scientific “Blabla”, I can only recommend “Das Liebesleben der Tiere (The Love Life of Animals)” by Katherina von der Gathen.
In a pedagogically meaningful and child-friendly way, without being obscene, the reproductive behavior of different animals is addressed, from mate choice to rearing of the offspring everything is there: funny, beautifully illustrated and oriented on facts.
Here are just a few extreme examples presented.
Do men have three legs?
The function of the genitals lies in the distribution or uptake of the germ cells. In amniotes (these include: mammals, birds, “reptiles”), the external genitalia develop from the cloaca. This is an embryonic organ, which is necessary for excretion. In amphibians, “reptiles”, birds and egg-laying mammals, it is a common body exit for the digestive, sexual and excretory organs. In viviparous mammals, the cloaca is indeed embryonic, but replaced by separate export openings (anus, urogenital tract). Some mammal groups, such as the Tenreks native to Madagascar, have once again developed a secondary cloaca from previously separate orifices (B. Riedelsheimer, et al., 2007).
But in all amniotes, including mammals, the embryonic cloaca induces the genitalia (Tschopp et al 2014, Herrera and Cohn 2014, Gredler et al., 2014, cf Fig. 1). The external genitalia begin as a swelling of cells, so-called genital tubercles (tuberculum genital), on both sides of the cloacal membrane. Corresponding experiments have shown that the lateral plate mesoderm is the source of the genital tubercles (tail cells in mice, cells of the tail and limb buds in chickens and cells of the limb buds in lizards, see Fig. 1). The mesoderm is one of the embryonic germ layers from which certain organs develop. Limb buds are the embryonic appendages of the limbs.
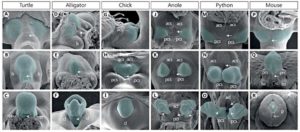
Fig.1: SEMs of Amniote External Genitalia Development Ventral views and anterior is at the top (D and G are lateral views with anterior to the left.) acl = Anterior cloacal lip; acs = anterior cloacal swelling; cl = cloacal lip; g = glans; ls = lateral swelling; lss = labioscrotal swelling; p = prepuce; pcl = posterior cloacal lip; pcs = posterior cloacal swelling; prs = preputial swellings. (From Gredler et al. 2014.)
The reason why different groups of mesoderm cells are being recruited for genitalia formation in different groups of amniotes has to do with the location of the cloaca. The cloaca forms in different places in different groups of amniotes. In mice, the cloaca extends caudally past the hindlimbs, so the genital tubercles are posterior to the limbs. In snakes, the genital tubercle is at level of hindlimb buds. In chicks, it extends between the limb bud and tail. And if a cloaca is transplanted into a chick embryo at different sites, the lateral mesoderm cells at that site will be recruited into the genital tubercle (Tschopp et al. 2014). In the chick and mice, the two genital tubercle cell populations fuse ventrally as the closure of the body wall brings the right and left sides of the embryo together . This allows the formation a single genital organ. In snakes, however, the limb bud cells are “re-purposed” as genitalia and remain near the sides. Thus, the cloaca acts as the signaling center for genitalia by secreting sonic hedgehog (Shh) and Fgf8 paracrine factors (Haraguchi et al. 2001). In the tail, this causes an epithelial-to-mesenchymal transition in the lateral mesoderm, and its migration to the ventral region. “Sonic Hedgehog” (Shh) is also important in limb development (Tickle & Towers 2017).
So now you know why snakes don’t have limbs. They’ve been recruited to forming its two copulatory organs (Figure 2). The two penises of the snake are called hemipenes. Both are usually functional, but not at the same time.
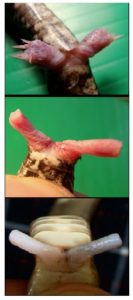
Fig. 2: Hemipenes of Ovophis monticola (Mountain Pit Viper), Pareas margaritophorus (Whitespot Snail Snake), and Naja siamensis (Siamese Speikobra). https://www.sjonhauser.nl/hemipenes-amazing-copulatory-organs-of-snakes.html
Adam’s Rib
The similarity between limbs and genitals is also evident by other findings. Most mammals (but not humans) have genital bones: Os clitoris in females and Os penis (penile bones, baculum) in males. These are retractable bones, which give the copulation organs stability. They also stimulate the sexual partner and protect the urinary tract (Herdina et al., 2015, Fig. 3). They are typical for rodents, predators, bats and primates. In some marine mammals, they can be quite large.

Fig. 3: Dog and human. While a baculum is present in dogs, it is absent in humans. https://www.smithsonianmag.com/smithsonian-institution/grover-krantz-donated-his-body-to-science-on-one-condition-38726179/
The development of the rodent baculum is regulated by a set of HoxD transcription factors (Zákány et al., 1997), which are induced by members of the TGF-b1 and BMP families (also transcription factors) (Origuchi et al., 1998). The Hox genes (important regulatory genes for the development of an organism), which are necessary for the development of the fingers and toes, are also found in the baculum (Lonfat et al., 2014). It is not known why humans lack the baculum. It may have something to do with the development of upright gait, making the baculum more susceptible to injury.
It is particularly interesting when we connect the missing baculum of man with the creation story of the Bible. Of course, the biblical story of creation is not literal, but it does provide interesting stories of how people looked at the world at that time. Anyone who knows Genesis’s story of creation knows that God formed Eve out of Adam’s rib. The “logical” problem is that Adam still has his full set of ribs. Thus, in a joint publication by the developmental biologist S. F. Gilbert and the Bible researcher Z. Zevit suggests that the rib of Adam, from which God creates Eve, actually was his penile bone. The rib would be a mistranslation of a Hebrew euphemism for penile bones (Gilbert & Zevit 2001). I cannot confirm or disprove the “correctness” of this interpretation (and it is not shared by all). But it is a good example that two different disciplines can cooperate with each other.
Kiss of the cloaca
Male birds differ enormously in their external genitals. For example, male chickens and ducks differ in penis size. Chickens have no real external phallus, which seems particularly puzzling when compared to the considerable penis of geese and ducks (McCracken et al 2001, Fig. 4). However, chickens are the norm, as less than 5% of all male bird species have a phallus capable of penetration (in addition to geese, such a phallus is found in ratites). The agent, which induces a shortening of the chicken phallus, is the transcription factor BMP4, which (similar to the formation of fingers) induces cell death (apoptosis) in the genital hump. In the period in which the phallus should develop, BMP4 is found in the corresponding cells in chickens, but not in ducks. Addition of the protein noggin to the genital humps of chickens inhibits (inhibits) the function of BMP4 (Herrera et al., 2013).
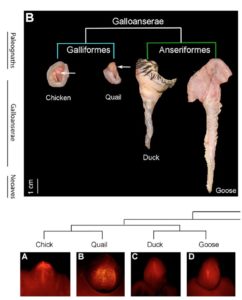
Fig. 4: Penis development in birds. As with most birds, chickens and quail have a very reduced penis incapable of penetration. Ducks and geese, on the other hand, have a bigger penis.
So that sperm can penetrate into the female, birds with a small phallus operate a so-called “kiss of the cloaca”. In the process, the male collects the semen in his cloacal opening and transfers it to the female cloacal opening, which positions itself and opens its vagina (Fig. 5). The origin of the rather vulgar used German word “vögeln” (derives from Vogel = bird; “vögeln” is a vulgar for mating) derives from Middle High German and actually referred to the copulation in birds and was later transferred to humans (Pschyrembel Dictionary Sexualität 2006, pp. 577-578).
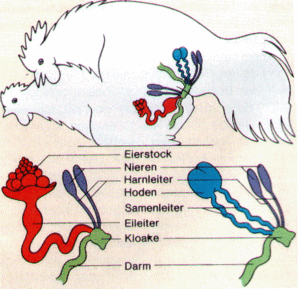
Fig. 5: Copulation in birds. With kissing the cloaca, the sperm penetrates into the female. Source: http://www.projekt.didaktik.mathematik.uni-wuerzburg.de/mathei/eibio/embryonalentwicklung.htm
A Real Butch
Normally, the hormone testosterone causes the genital tubercle of the embryo to form into a penis (Rodriguez et al., 2012). However, hormonal (or genetic) disorders can lead to a different picture, one speaks of intersexuality. But this intersexuality seems to be “normal” in the spotted hyena (Crocuta crocuta), because female hyenas have a clitoris similar in length and shape to a penis. Female spotted hyenas are the only mammal species that use their clitoris not only for copulation but also for birth; they also lack a vaginal opening, (Fig. 6). The animals are not by definition “intersexual” but clearly female because they are a) fertile and b) produce female germ cells. In this species, the growth of the penis and clitoris does not depend on the testosterone, although details of the anatomical differences are testosterone dependent. However, the mechanisms are not fully understood yet (Cunha et al., 2014).
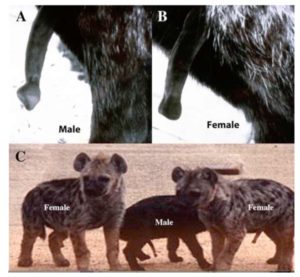
Figure 6. The adult erect hyena penis (A) and clitoris (B). While both are large and extend from the body, they can be recognized by the distinctive shapes at the tip. (C) Three infant spotted hyenas (3-4 months old) in full erection. (From Cunha et al. 2014.)
In hyena females are more dominant and aggressive than males and the also have higher testosterone levels. A long-term study on spotted hyenas of Ngoro-Ngoro Crater in Tanzania, where since 1996 8 hyena clans (up to 130 animals per clan) were observed and 4133 interactions, with 748 hyenas involved, were able to find out that the higher aggression and the higher levels of testosterone alone are not enough to ensure the dominance of females. A much greater role was played by social support from other clan members. When two hyenas come into conflict with each other, regardless of sex or height, it is the support of other hyenas that determine the winner. The body mass has virtually no influence. Sex plays a role in that the social system is based on a cooperation of the females. But sex alone is not enough to be the winner. Females dominate through their social support, which is much easier due to their matrilineal organization: females rarely leave the clan where they were born, only the males migrate away. As a result, females have a higher chance of being higher than males, since newly immigrated males in a clan need the support of females (Vullioud et al., 2018). A summary of the work can also be found here:
Doubtless hyenas are really cool animals and by far not the stupid and lazy scavengers or baddies, but rather more successful than lions in hunting.
“Pregnant” Men …
Seahorses (genus Hippocampus) are already strange fish. Not only does their body shape differ from those of other “typical” fish, but their mating behavior as well. The males carry under their tail a pouch provided with an opening at the top. During the mating season, they swim around the females, stretching their heads and pumping their pouch with water and open it this way.
During a sustained mating ritual, the partners swim together to the water surface, embrace there with the tail and rotate in a circle for some time; while the female puts up to 200 eggs in the pouch of the male. Once arrived, they will be fertilized (a couple may have offspring up to 15 times a year). But the males do not become pregnant and are clearly male. They produce sperm, fertilize the eggs and just carry them in their pouch (Millus 2000, Robinson 2013, Masonjones & Lewis 1996, Fig. 7).
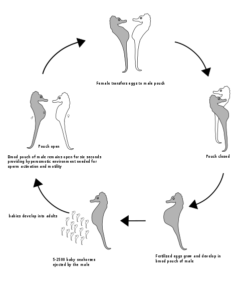
Fig. 7: Mating behavior of seahorses. Source: wikipedia
Shemale Fishes
Some species have been documented to be able to change sex in certain circumstances. This is the case in the Tobacco fish (Serranus tabacarius). This species is an example of simultaneous hermaphroditism, they can act from one moment to the next as a male and then as a female. When two animals meet and are in a mating mood, one of the fishes begins to take on the role of the female and thus initiates the courtship behavior. Once mating is complete, they swap roles (and sexes, Petersen 1995, 2006).
In addition to the simultaneous hermaphroditism, there is also the sequential one. Here, the organism function as one sex, but certain conditions cause a female animal to turn into a male or vice versa, such as the bluehead wrasse (Thalassoma bifasciatum). These Caribbean coral fish have one female type but two alternative male types adapted for two different social conditions. Imagine living as a youthful bluehead wrasse on a sparsely populated coral reef. In such circumstances, a few male, beautifully colored bluehead wrasse defends the few nesting sites and mate with the females. In such highly competitive areas few males monopolize the area and it is therefore useful for the young bluehead wrasse to develop as a female. You can grow, become taller and, if necessary, become a male when the opportunity arises. This can happen as soon as one of the males dies and one changes the sex quickly and changes from female to male. But this change of sex can take a few days and, in this time, other females can turn into a male, of which only one can dominate the breeding area. Consequently, the sex change of the other females must be suppressed and this works best when one of the females behaves like a dominant male. Because the change in behavior is progressing much faster than the physical change their brain can create this male behavior without having to convert the ovaries into testicles. Once the male behavior is achieved, the fish can wait for the testicles to develop. These females develop into secondary males because the testes of the animals used to be ovaries. This happens under conditions of low population density. The primary males who first developed their testes are smaller and less colorful than the secondary males. They occur in heavily populated areas, where there are many bluehead wrasses (Munday et al., 2006, Semsar & Godwin 2003). A change of sex has also been documented in some frog species, e.g. in reed frogs (Hyperolius viridiflavus) from Central Africa (Grafe & Linsenmair 1989).
Another species of fish, the bluegill (Lepomis macrochirus), has two different types of males. The “parent-males” reach their sexual maturity at the age of seven and are quite tall. They build nests and defend them against invaders. If a female comes to the nest, she spawns her eggs, which are fertilized by the male. The survival of the fertilized eggs depends on the protection of the males. In addition to these parental males, there are so-called “satellite males”. They become sexually mature at the age of two and are much smaller and faster. They fertilize eggs by invading a nest and release their sperm. In order not to be attacked and expelled by the “parent-male” they have the same color as the females (Neff et al., 2003).
Men are parasites!
In some fish species, the sex difference is quite large. A good example are some species of deep-sea angler fish, where the females can be many times bigger than the males! In the species Ceratias holboelli the females are 60 times as long and half a million times heavier than the males (see Fig. 8).
When a male with his big eyes and nostrils finds a female, he bites into the female. Once bitten into the female, his individuality also disappears as the body and blood vessels merge. It provides the male with nutrients and oxygen; he provides therefore the sperm. We are dealing here with a so-called sexual parasitism (Pietsch 2005).

Fig. 8: Ceratias holboelli, very large female and on the ventral side (middle) the very small male. Source: wikipedia
If male angler fishes are really small, they have almost disappeared with the Amazon molly (Poecilia “formosa“). Only females are known from this type of fish occurring in Texas and Mexico. This species was originally created by the hybridization of two closely related species (Poecilia mexicana and Poecilia latipinna). Actually, the Amazon molly produces diploid eggs, so it reproduces asexually. Interestingly, however, they require the sperm of closely related species to initiate the division of the ovum. The gene material of the sperm cell is not used! (Lampert & Schartl 2008). This form of asexual reproduction, in which the egg is made to divide by contact with a sperm cell, without using the genetic material of the sperm cell, is called gynogenesis.
Who needs men anyway?
Some species of American racerunners and whiptails lizards (Teiidae) have only females. They propagate asexually (so-called parthenogenesis) and only females hatch from the eggs (Maslin 1962).
Such parthenogenesis has also been observed with Komodo dragons (Varanus komodoensis), at least in zoos. Here, however, there is the peculiarity that after the storage of such eggs only males hatch. This is related to the ZW system of sex determination that occurs in monitor lizards. In the case of monitor lizards, parthenogenesis apparently produces only animals with a ZZ-chromosome set (males) or with a set of WW-chromosomes which are nonviable (Watts et al., 2006).
When bacteria affect your sex
But it is even more absurd: Wolbachia is a parasitic bacterial genus living mainly insects, but also affects other arthropods and nematodes. It is estimated that 25-70% of all insect species are affected by this bacterial species (Taylor 2018, Kozek & Rao 2007). They are also known to often live in the genitalia of their hosts and thus influence their reproduction. They prefer egg cells but not sperm cells. In order to promote the spread of Wolbachia, this bacterial genus has developed a number of strategies (see Fig. 9):
– Killing infected males during larval development, thereby increasing the rate of born and infected females (Hurst et al., 1999).
– Feminization of infected males, which is common in butterflies (Fuji et al., 2001).
– Promotion of parthenogenesis. This is in the wasp genus Trichogramma, which propagates without males through the presence of Wolbachia (Knight 2001, Murray 2009)
– Prevention of fertilization of uninfected females by cytoplasmic incompatibility (Breeuwer & Werren 1990)

Fig. 9: left: Wolbachia bacteria within an insect cell, right: Influence of Wolbachia on reproduction
“Horny” without horn
The superfamily Scarabaeoidea is with 14 families, 2000 genera and about 35,000 species a very diverse group of beetles, which include some particularly spectacular species: rhinoceros beetle, Hercules beetle, cockchafer, scarab beetle, rose beetle, stag beetle, Goliath beetle, etc. Here you can find not only the largest beetle species (Hercules beetle, Goliath beetle), but also many species with spectacular horns. More specifically, the males are proven with horns and shields and fight for females.
In one species, Onthophagus taurus, there are males with horns and without horns (the females are always without horns, see Fig. 10). Dung is needed for the development of the larvae. The females dig gross tunnels under dung heaps and transport parts of the dung into the tunnels. Dung balls are formed and are placed at the end of the tunnel. Then a small egg chamber is dug into the dung ball, into which a single egg is laid. The egg chamber is closed and the egg then left to itself. The entire nourishment of the larva consists of the dung ball shaped for it. The males use their horns in aggressive battles for access to the tunnels of the females. The winner is the owner of the gross tunnel and is allowed to mate with the female. Horned males usually stay with a female, guard it and help complete the tunnel. Males which have no or stunted horns use a different strategy. They hide at the entrance of already occupied gross tunnels and sneak into the tunnels to the females when their partner is just outside the tunnel. Or they hide in the sometimes heavily branched tunnel system, thus avoiding the dominant male. Due to the lack of horns, they move much more skillfully than their horned competitors. The mating may also take place outside the tunnel when the female is collecting dung for the brood. In this way, even smaller males have success in mating (Moczek 1999, Moczek & Emlen 2000).

Fig. 10: Male and female beetles in Onthophagus taurus. Source: http://extendedevolutionarysynthesis.com/conditional-gene-expression-developmental-plasticity-diversification-horned-beetles/
The question that still remains open is: How do Ontophagus taurus males develop with horns and how without? Several studies have shown that the nutritional factor plays an important role, which influences the hormone system and gene expression. It is an epigenetic process (Moczek 2017, Kijimoto & Moczek 2016, Kijimoto, Moczek & Andrews 2011, Hunt & Simmons 2000).
These examples – and there are many more – from the animal kingdom have shown the many forms of sexual development. But how varied the forms may be, there are always two sexes, even though some species have two types of males, these two types stay males.
Literature
Cunha, G. R. and 17 others. 2014. Development of the external genitalia: perspectives from the spotted hyena (Crocuta crocuta). Differentiation 87(1–2): 4–22.
Fujii, Y.; Kageyama, D.; Hoshizaki, S.; Ishikawa, H.; Sasaki, T. (2001-04-22). “Transfection of Wolbachia in Lepidoptera: the feminizer of the adzuki bean borer Ostrinia scapulalis causes male killing in the Mediterranean flour moth Ephestia kuehniella”. Proceedings of the Royal Society of London B: Biological Sciences. 268 (1469): 855–859.
Gilbert, S. F. and Z. Zevit. 2001. Congenital human baculum deficiency: the generative bone of Genesis 2:21-23. Am J Med Genet. 101(3): 284–285.
Grafe, T. U.; K. E. Linsenmair (1989). “Protogynous sex change in the reed frog: Hyrperolius viridiflavus”. Copeia. 4 (4): 1024–1029.
Gredler, M. L. and 7 others. Evolution of External Genitalia: Insights from Reptilian Development. Sex Dev. 8(5): 311–26. doi: 10.1159/000365771.
Haaretz.com (2015): Why God Didn’t Use Adam’s Penis Bone to Make Eve
Haraguchi et al. 2001: Unique functions of Sonic hedgehog signaling during external genitalia development. Development 2001 128: 4241-4250;
Herdina, A. N., D. A. Kelly, H. Jahelková, P. H. Lina, I. HoráČek and B. D. Metscher. 2015. Testing hypotheses of bat baculum function with 3D models derived from microCT. J Anat. 226(3): 229–235.
Herrera, A. M., S. G. Shuster, C. L. Perriton and M. J. Cohn. 2013. Developmental Basis of Phallus Reduction during Bird Evolution. Current Biology. 23(12): 1065–1074.
Herrera, A. M. and M. J. Cohn. 2014. Embryonic origin and compartmental organization of the external genitalia. Sci Rep. 2014, Nov. 5; 4: 6896.
Hunt, J., & Simmons, LW (2000): Maternal and paternal effects on offspring phenotype in the dung beetle Onthophalgus taurus. Evolution 54, 936 – 941
Hurst G., Jiggins F. M., Graf von Der Schulenburg J. H., Bertrand D., et al. (1999). “Male killing Wolbachia in two species of insects“. Proceedings of the Royal Society B. 266 (1420): 735–740.
Teiya Kijimoto, Armin P. Moczek, Justen Andrews (2011): Diversification of doublesex function underlies morph-, sex-, and species-specific development of beetle horns PNAS December 11, 2012 109 (50) 20526-20531
Teiya Kijimoto and Armin P. Moczek (2016): Hedgehog signaling enables nutrition-responsive inhibition of an alternative morph in a polyphenic beetle PNAS May 24, 2016 113 (21) 5982-5987;
Knight J (5 July 2001). “Meet the Herod Bug“. Nature. 412 (6842): 12–14.
Kozek, Wieslaw J.; Rao, Ramakrishna U. (2007). “The Discovery of Wolbachia in Arthropods and Nematodes – A Historical Perspective“. Issues in Infectious Diseases. Issues in Infectious Diseases. 5 (Wolbachia: A Bug’s Life in another Bug): 1–14.
K.P. Lampert, M. Schartl: The origin and evolution of a unisexual hybrid: Poecilia formosa. Philos Trans R Soc Lond B Biol Sci. 2008 Sep 12;363(1505):2901-9.
Lonfat, N., T. Montavon, F. Darbellay, S. Gitto and D. Duboule. 2014. Convergent evolution of complex regulatory landscapes and pleiotropy at Hox loci. Science 346(6212): 1004–1006.
Maslin, P. T. (1962): All-Female Species of the Lizard Genus Cnemidophorus, Teiidae Science 19 Jan 1962: Vol. 135, Issue 3499, pp. 212-213
Masonjones, Heather D.; Lewis, Sara M. (1996). “Courtship Behavior in the Dwarf Seahorse, Hippocampus zosterae”. Copeia. 1996 (3): 634–640.
Milius, S. (2000). “Pregnant: And Still Macho” (PDF). Science News. 157 (11): 168.
Armin P. Moczek: Facultative paternal investment in the polyphenic beetle Onthophagus taurus: the role of male morphology and social context. In: Behavioral Ecology. 1999. Volume 10, Issue 6, pp. 641-647.
Armin P. Moczek (2017): Conditional gene expression, developmental plasticity, and the diversification of horned beetles
Armin P. Moczek, Douglas J. Emlen: Male horn dimorphism in the scarab beetle, Onthophagus taurus: do alternative reproductive tactics favour alternative phenotypes? In: Animal Behaviour. 2000. No. 59, 459-466.
Munday, PL, et al. (2006): A social basis for the developement of primary males in a sex-changing fish. Proceeding of the royal society of London B 273, 2845 – 2851
Murray, Todd. “Garden Friends & Foes: Trichogramma Wasps”. Weeder’s Digest. Washington State University Whatcom County Extension. Archived from the original on 2009-06-21. Retrieved 16 July 2009.
Neff, BD, Fu, P., Gross MR (2003): Sperm investment and alternative mating tactics in bluefull sunfish (Lepomis macrochirus). Behavioral Ecology 14, 634 – 641.
Origuchi, N., Y. Ishidou, T. Nagamine, T. Onishi, S. Matsunaga, H. Yoshida and T. Sakou. 1998. The spatial and temporal immunolocalization of TGF-beta 1 and bone morphogenetic protein-2/-4 in phallic bone formation in inbred Sprague Dawley male rats. In Vivo 12(5): 473–480.
Petersen CW. Reproductive behavior, egg trading, and correlates of male mating success in the simultaneous hermaphrodite, Serranus tabacarius, Env Biol Fish, 1995, vol. 43 (pg. 351-61)
Petersen CW. (2006): Sexual selection and reproductive success in hermaphroditic seabasses Integrative and Comparative Biology, Volume 46, Issue 4, 1 August 2006, Pages 439–448,
Pietsch, Theodore W. (25 August 2005). “Dimorphism, parasitism, and sex revisited: modes of reproduction among deep-sea ceratioid anglerfishes (Teleostei: Lophiiformes)“. Ichthyological Research. 52 (3): 207–236.
Riedelsheimer, Pia Unterberger, H. Künzle und U. Welsch: Histological study of the cloacal region and associated structures in the hedgehog tenrec Echinops telfairi. Mammalian Biology 72, 2007, S. 330–341
Robinson, James L (2013). Seahorses.
Rodriguez, E. Jr. and 8 others. 2012. Specific morphogenetic events in mouse external genitalia sex differentiation are responsive/dependent upon androgens and/or estrogens. Differentiation 84: 269–279.
Semsar, K. & Goldwin, J (2003): Social influences on the arginine vasotycin system are independent of gonads in sex-changing fish. Journal of Neuroscience 23, 4386 – 4393
Taylor, M.J. (2018). “Microbe Profile: Wolbachia: a sex selector, a viral protector and a target to treat filarial nematodes”. Microbiology 164: 1345-1347
Tickle, C. & Towers, M. (2017): Sonic Hedgehog Signaling in Limb Development Front Cell Dev Biol. 2017; 5: 14.
Tschopp, P. and 8 others. 2014. A relative shift in cloacal location repositions external genitalia in amniote evolution. Nature 516(7531): 391–394.
Vullioud, E. Davidian, B. Wachter, F. Rousset, A. Courtiol & O.P. Höner, “Social support drives female dominance in the spotted hyaena,” Nature Ecology & Evolution, 2018.
C. Watts et al. (2006): Parthenogenesis in Komodo dragons. Nature 444, S. 1021–1022,
Zákány, J., C. Fromental-Ramain, X. Warot and D. Duboule. 1997. Regulation of number and size of digits by posterior Hox genes: a dose-dependent mechanism with potential evolutionary implications. Proc Natl Acad Sci U S A. 94(25): 13695–700.